7. Alcoholic Beverages
Alcoholic fermentations have been well known since ancient times, though little was known of the nature of the process. Louis Pasteur could show that yeasts were the organisms responsible for such a process. Later details became available about the chemistry of fermentations, which were exploited for the industrial manufacture of alcoholic beverages. Microorganisms, principally, yeasts in the genus Saccharomyces are used to produce various types of alcoholic beverages. The process relies on alcoholic fermentation conversion of sugar to alcohol by microbial enzymes. (BSc Industrial Microbiology Notes & Study Material)
1. Beer. The fermentation of beer is usually a batch process. In some countries, the production of beer is carried out in a continuous flow-through process. Beer is a product of the fermentation of barley grains by yeasts. Barley seeds are allowed to germinate. During germination, the naturally occurring amylases convert the grain starch to sugars, most of which are maltose. The process is called malting and the digested grain is malt. The next step is to mash the grain with water and remove the fluid portion called the wort.
Dried petals of a vine, Humulus lupulus, called hops are then added to the wort to give it flavor, colour, and stability. Hops also prevent contamination of the wort, due to the presence of two antimicrobial substances in petals. At this stage, the fluid is filtered and yeast is added in large quantities. (BSc Industrial Microbiology Notes & Study Material)
The yeast, commonly used in the fermentation of wort is one of the many strains of Saccharomyces cerevisiae, developed by brewers. Generally, the yeast is taken from a previous batch of beer. Some yeasts give uniform cloudiness to the beer and are carried to the top of the fermentation vat by foaming carbon dioxide. Such yeasts are called top yeasts and the product is ale. Other yeasts ferment the beer more slowly and produce less alcohol than ale. These yeasts are known as bottom yeasts and their product is lager beer. Saccharomyces carlsbergensis, used in U.K. is a bottom fermenter. (BSc Industrial Microbiology Notes & Study Material)
Generally, one week’s time is needed for normal fermentation to take place. After a week, the young beer is transferred to vats for primary and secondary aging. It may take about six months more. Some yeast is left in the beer that is to become keg beer and the product is refrigerated to preserve it. The thick wall of the keg traps CO2 produced during continual fermentation. For canning or bottling the beer is to be pasteurized at 140°F for 13 min. to kill the yeast or may be filtered to remove the yeasts. Some yeast is used to seed new wort, the rest for animal feed or pressed to tablets for human consumption (single cell protein or SCP). The alcoholic content of beer is roughly 4 percent.
Sake, though commonly known as rice wine, is somewhat like a rice beer. It is obtained from steamed rice. The rice starch is converted first to sugar by Aspergillus oryzae or Rhizopus sp. Later Saccharomyces spp. ferment the sugar until the alcohol level is approximately 14 percent when the sake is ready for consumption. (BSc Industrial Microbiology Notes Study Material)
2. Wine. Wine fermentations are carried by controlled cultures of Saccharomyces ellipsoids, a variety of Saccharomyces cerevisiae, or with the strains of yeast naturally occurring on grapes. The former method is common in the U.S.A., the latter in Europe.
Wine is made from ripe fruit, fruit juice, or plant extract such as dandelions. Fermentation usually begins with the crushing of the fruit to produce must. Sulfur dioxide may be added to control the process. In natural fermentations, SO2 is not used, and the yeasts begin to digest sugar. Oxygen may be supplied to promote the aerobic growth of the yeast. However, anaerobic conditions are established later.
Alcohol production occurs within a few days, though aging may take months or years. During this period, secondary fermentations develop the flavour, aroma, and bouquet of the wine. Red wine becomes red as alcohol extracts the colour of grape skin. For red wine, fermentation is carried out at 24-27°C for 3-5 days and white wine takes 1-2 weeks at 10-21°C. Additional carbon dioxide production yields Champagne and other sparkling wines which are naturally carbonated. Sherry wines result from inoculation with special yeasts to have unique flavours.
In dry wines, most or all of the sugar is metabolized, whereas, in sweet wines, the fermentation is stopped before the entire sugar is consumed. The strongest natural wines have about 16 percent alcohol (yeasts cannot tolerate higher levels than this). Most table wines average about 10-12 percent alcohol, with fortified wines reaching 22 percent alcohol. In fortified wines, brandy or other spirits are added to produce port, sherry, and cocktail wines. For mass production, the wine is pasteurized, filtered, and bottled.
3. Distilled spirits. They contain considerably more alcohol than beer or wine. The alcoholic content is shown by a proof number which is twice the actual percentage of alcohol. The process of the distilled spirit begins with the same type as for wine and beer, except that after the fermentation process the alcohol is collected by distillation, to allow a higher concentration of alcohol. The raw product is first fermented by Saccharomyces, then aged, and finally matured in casks. At this point, the process differs. The alcohol is concentrated by a distillation apparatus using heat and vacuum. (BSc Industrial Microbiology Notes Study Material)
During further maturing unique flavours from the chemicals such as aldehydes, ethers, and volatile acids are added. The alcohol content is then standardized by diluting it with water before bottling it. There are four basic types of spirits: brandy is made from fruit juice, rum from molasses, whisky from malted cereal grains, for instance, scotch from barely, rye from rye grain, and bourbon from corn. Neutral spirits, such as vodka, are made from potato starch and left unflavoured, and gin flavoured with juniper oil.
8. Vinegar
Vinegar production involves an initial anaerobic fermentation to convert carbohydrates by Saccharomyces cerevisiae to alcohol, followed by a secondary oxidative transformation of the alcohol to form acetic acid by Acetobacter and Gluconobacter. The starting materials for vinegar may be fruits (grapes, oranges, apples, pears), vegetables (potatoes), malted cereals Charley, rye, wheat, corn) and sugary syrups (molasses, honey, maple syrup). Wine vinegar comes from grapes and cider vinegar from other fruits. In slow methods of vinegar production, still used in some European countries, an initial natural alcohol fermentation achieves an alcohol concentration of 11-13%.
After producing the alcoholic liquid, acetic bacteria are seeded into the solution and allowed to convert the alcohol slowly to acetic acid. In the Orleans process, a barrel is filled about 1/4th full with raw vinegar from a previous run to provide the active inoculum. A wine, hard cider, or malt liquor is then added as a substrate. Air is left in the barrel, acetic acid bacteria grow as a film on the top of the liquid, and conversion to acetic acid occurs in several weeks to several months to complete at 21-29°C.
To increase the rate of acetic acid production, a vinegar generator can be used in which alcohol-containing liquid is trickled over a surface film of acetic acid bacteria. The film of bacteria is maintained on wood chips. Alcohol liquid is sprinkled over the wood chips and during this slow-trickling the liquid down through the generator, the alcohol is converted to sc acid. Air enters the generator from the bottom.
Today, though in the industrial production of vinegar submerged culture reactors are used, forced aeration is used to maximize the rate of acetic acid production. The bacteria grow in the fine suspension created by air bubbles and the fermenting liquid. Here 8-12% alcoholic liquid is inoculated with Acetobacter sp. at 24-29°C with controlled aeration. The vinegar thus formed is clarified by passing through a filter and allowed to age for taking a final taste. (BSc Industrial Microbiology Notes Study Material)
9. Enhanced Recovery of Metals (Bioleaching)
Since high-grade ore deposits are easily accessible, these become rapidly depleted. It thus becomes necessary to recover mineral resources from low-grade ore deposits. However, no appropriate technology is still available for the recovery of metals from low-grade deposits. It is encouraging to find some microorganisms that could do it efficiently. This potential of microbes could only be realized recently and efforts are being made to use them for enhanced recovery of mineral resources from natural deposits. Microbes have been used for the recovery of two important natural resources – metals and petroleum. (BSc Industrial Microbiology Notes Study Material)
It was in 1957 that a relationship between the presence of Thiobacillus ferrooxidans and the dissolution of metals in copper-leaching operations was recognized by American microbiologists. T. ferrooxidans and T. thiooxidans are thermoacidophilic archaebacteria. They are autotrophs and grow in acidic and hot environments. It has been demonstrated that this Thiobacillus spp. can be used for the extraction of copper and uranium from insoluble minerals. (BSc Industrial Microbiology Notes Study Material)
This implication of microbial activity in weathering, leaching, and deposition of mineral ores could develop into a recent field of biotechnology – biohydrometallurgy. Biomineralisation is the deposition of metals as insoluble oxides and sulphides due to microbial activity.
Microbial mining is the process of bioleaching recovering metals from ores that are not suitable for direct smelting due to their low metal content. Bioleaching uses microbes to alter the physical or chemical properties of a metallic ore so that the metal can be extracted. Metals can be extracted economically from low-grade sulphide or sulphide-containing ore by exploiting the metabolic activities of thiobacilli, particularly T. ferrooxidans. Under optimal conditions in the laboratory, as much as 97% of the copper in low-grade ores has been recovered by bioleaching, but such high yields are not achieved in actual mining operations.
The process is at present commercially used for the recovery of copper and uranium from low-grade ores. Laboratory experiments could show that recovery of other metals such as Ni, Zn, Co, Sn, Cd, Mb, Pb, Sb, As, and Se from their low-grade sulphide-containing ores is also possible through bioleaching. The leaching process can also be used to separate the insoluble lead sulphate (PbSO4) from other metals that occur in the same ore. (BSc Industrial Microbiology Notes Study Material)
The general process carried out by T. ferrooxidans (T.f.) and related species can be shown by the following equation.
MS + 202 → MSO4 where M is divalent metal.
Because metal sulphide is insoluble and metal sulphate usually water-soluble, this transformation produces a readily leachable form of the metal. T.I., a chemolithotroph derives energy through the oxidation of either a reduced sulphur compound or ferrous iron. It exerts its bioleaching action by oxidizing the metal sulphide being recovered either directly converting S2- to SO42- and/or indirectly by oxidizing the ferrous iron content of the ore to ferric ion. The ferric ion, in turn, chemically oxidizes the metal to be recovered to a soluble form that can be leached from the ore. (BSc Industrial Microbiology Notes Study Material)
It is possible to leach the ore in situ without first mining it if its formation is porous and overlays a water-impermeable stratum. A pattern of boreholes is established with some of the holes used for injecting the leaching liquor and others for the recovery of leachate. More frequently, however, this bioleaching process is used after the ore is mined, broken up, and piled in heaps on a water-impermeable formation or on a specially constructed apron. Water is then pumped to the top of the ore heap and trickles down through the ore to the apron. A continuous reactor leaching operation for recovery of copper from its low-grade sulphide ore is shown in Fig.
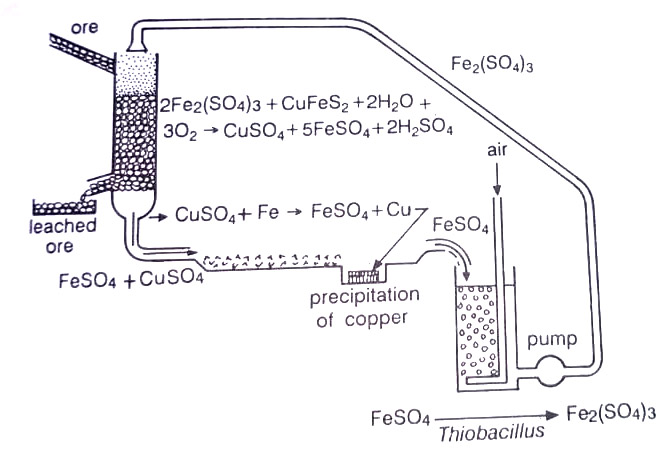
The leaching water and ore usually supply enough dissolved mineral nutrients required by T.f., but in some cases, NH3 and PO4 may be added. The leached metal is extracted with an organic solvent and then removed from solvent by stripping. Both the leaching liquor and the solvent are recycled. (BSc Industrial Microbiology Notes Study Material)
Copper is generally in short supply. Low-grade copper ore contains 0.1-0.4% Cu. The pregnant leaching solution may contain 1 to 3 g of Cu/l. In copper leaching operations, Thiobacillus involves both, direct oxidation of CuS and indirect oxidation of CuS via the generation of ferric ions from ferrous sulphide, present in most of the important copper ores such as chalcopyrite (CuFeS2). In the latter case, copper replaces iron i.e. CuSO4 + Fex → Cux + FeSO4. In the 1980s various firms began to utilize bioleaching for extracting copper.
The recovery of uranium, a nuclear fuel, can also be enhanced by microbial activities, which should help overcome the global energy crisis. Moreover, the current controversies about nuclear plants may also be diluted/solved, at least from an economic point of view, if not safely. Insoluble tetravalent uranium oxide (UO2) occurs in low-grade ores. There is no evidence for direct oxidation. But UO2 can be indirectly converted to leachable hexavalent form (UO2SO4) by T.f., which oxidizes ferrous iron in pyrite (FeS), which often accompanies uranium ores.
The oxidized iron as an oxidant converts UO2 to UO2.SO4 chemically, which can be recovered by leaching. In Canada, bioleaching was first employed in 1970 for the extraction of uranium. (BSc Industrial Microbiology Notes & Study Material)
Recovery of copper and uranium through bioleaching depends on several factors, such as type of the geological formations, ore characteristics, and prevailing conditions under which the concerned microbe is to grow. Also, the oxidative activity of Thiobacillus results in high temperatures, and other bacteria like Sulpholobus (obligate thermophile and acid tolerant) can be useful that can oxidise ferrous iron and sulphur in a manner similar to thiobacilli. Sulpholobus has been used for bioleaching of molybdenite (molybdenum sulphide), whereas Thiobacillus is intolerant of high concentrations of molybdenum, mercury, and silver.
Besides bioleaching, some microbes including fungi are able to accumulate metals in their cells at concentrations higher than in the surrounding media. Such bioconcentration has the potential for extracting rare metal ores from dilute solutions and for the recovery of metals (gold, silver) from industrial effluents. Rhizopus binds uranium from low-grade ores and nuclear wastes. Theoretically, microbes could be used to recover gold from the sea. In South, Africa efforts are being made to extract gold through bioleaching.
10. Enhanced Recovery of Petroleum
Besides metals, microbes can also be used to enhance the recovery of petroleum hydrocarbons. The tertiary recovery of petroleum (the use of bio. logical and chemical means to enhance oil recovery), and the enhanced recovery of hydrocarbons from oil shales are important due to depletion of recoverable oil resources. Tertiary recovery of oil uses solvents, surfactants, and polymers to dislodge oil from geological formations. Xanthan gums produced by some bacteria, such as Xanthomonas campestris, are useful compounds in oil recovery.
These polymers have high viscosity and flow characteristics that allow them to pass through small pores in rock layers containing oil deposits. Xanthan gums are added during water flooding operations (water is pumped into oil reservoirs to force out oil). This help pushes the oil toward the production wells. The polymers are produced by conventional fermentation in which X. compestris is grown and the gums are recovered. Many oil shales contain large amounts of carbonates and pyrites and their removal increases the porosity of shale, enhancing recovery of oil. The acid dissolves the carbonates and these can be produced by Thiobacillus spp. growing on sulphur and iron in the pyrite.
Thus bioleaching of oil shales by microbes has also the potential for enhancing the recovery of hydrocarbons.
Recombinant DNA Technology and Industrial Microbiology
It is genetically possible to “tailor” the microorganisms for the production of any microbial metabolite-vitamin, amino acid, or enzyme. Gene cloning extends the genome of the microorganism by allowing the introduction of novel genes from comparatively unrelated species.
The cloning of genes from higher eukaryotes, particularly from man and his domestic animals has been seen to offer even greater industrial potential. Which microbes should then be used as universal recipients for such genes and hence as production organisms? The two most ideal are the prokaryote, Escherichia coli, and the eukaryote, Saccharomyces cerevisiae. Some of the important products which gene cloning may make available in near future are as follows:

The above proteins could be obtained on large scale through fermentation by methods, relatively cheaper than the conventional ones. For example, human growth hormone was previously extracted from the pituitary gland of cadavers and was mostly in short supply. Now, an increase in supply should help more patients. Equally important is the development of new vaccines through gene cloning. Genes for single antigens can be cloned and expressed by bacteria and a purified antigen that has not been derived directly from the pathogenic organism or virus may be used as a vaccine. In this way, vaccines for viral hepatitis and foot-and-mouth disease have been developed.
Immobilisation of Enzymes and Cells – Relevance to Industrial Microbiology
Besides gene cloning, in microbial biotechnology several commercial processes have used immobilized microbial cells and enzymes in the last few years. Enzymes have been used in the industry for over 70 years, initially in detergents that are still perhaps one of the largest bulk users of proteases and lipases. In conventional biological industries also microbial enzymes have been used, e.g. proteases and amylases in malting and rennet in cheese manufacture. However, during the last 15 years or so, immobilized cells and enzymes have been used as production systems. Why enzymes are preferred over chemical processes?
(1) Enzymes carry out stereospecific reactions with high accuracy, whereas chemical technology results in many side-products from which the desired product is to be purified.
(2) Enzymes are cheap and carry out reactions at low temperatures and at atmospheric pressure, whereas chemical catalysts require special expensive conditions.
(3) Since there is much diversity in the microbial world, an enzyme for a desired reaction can be easily found by merely screening a range of microbes.
(4) Mutants with altered enzyme function can be isolated with appropriate genetic methods. Thus enzymes having different substrate specificities or with different physical properties (such as temperature resistance) can be isolated. Also, conventional genetics and gene cloning can be used for making specific changes in genes to increase the expression of the desired enzyme.
More recently, whole microbial cells have been used for specific chemical transformations. This should not be confused with anaerobic fermentation or conventional secondary metabolite production. Here only part of the cell’s metabolism is now being utilized, usually a single pathway, and sometimes only a single enzyme. The advantage of using cells is that the expense of purifying the enzyme is avoided and, in some cases, the enzyme is more stable in its natural environment than after purification. Frequently, cells and enzymes are subjected to immobilization on an inert support. (BSc Industrial Microbiology Notes & Study Material)
The stability of an enzyme is improved after its immobilization. Immobilization affords a simple way of separating the enzyme or cell from the products when the reaction is complete and is likely to prove invaluable in the development of biological sensors (biosensors) – special electrodes based upon the selectivity and high affinity of enzymes for their substrates.
In recent years the technology of enzyme and whole cell immobilization could have much impact on the industrial production of several products. Immobilization means “imprisonment or confinement of a biocatalyst in a distinct phase to a suitable inert support, where it can act upon its natural substrate repeatedly and continuously, and can be removed conveniently”. For a biocatalyst (enzyme/cell) the substrate is disposed of in a bulk phase. The physically entrapped or covalently bonded biocatalyst is chemically bonded to an inert, insoluble matrix (support), which is a high molecular weight polymer, such as glass beads, starch, cellulose, and polyacrylamide.
Since 1916, when the phenomenon was reported for the first time by J.M. Nelson and E.G. Griffin, this has been largely used in several manufacturing processes. They had initially reported the immobilization (adsorption) of invertase on charcoal/alumina without loss of activity. It was during the 1950s and 1960s that the technique became very popular.
Why immobilization?
Immobilization of enzymes has some advantages over free enzymes. These are (i) improved stability of the enzyme due to its binding to support, (ii) recovery of the enzyme at the end of reaction for repeated use, (iii) better efficiency of enzyme and manipulations of the catalyzed reactions, and (iv) higher purity and yield of product with conservation of resources and minimizing pollution risks.
Why whole cells?
Sometimes the use of immobilized enzymes becomes troublesome due to some reasons. For instance, the extraction and purification of enzymes can be quite expensive and tedious. Some reactions are catalyzed by more than one enzyme, thus with more enzymes, it becomes difficult to manage the reactor conditions. Moreover, immobilised enzymes can not be used where the enzyme needs continuous cycling of cofactors. Hence these problems can be overcome by using whole cells. (BSc Industrial Microbiology Notes Study Material)
How to immobilise?
A number of methods have been developed for the immobilization of enzymes, which except a few can also be used for cells as well. Basically, it is (i) entrapment, or (ii) binding. Entrapment can be accomplished by (i) fiber, (ii) microencapsulation, or (iii) gel. In gel entrapment, the processes involved are (i) polymerization, (ii) ionic network formation, or (iii) precipitation. Binding may be (i) cross-linking, or (ii) carrier-binding. In carrier-binding, the processes involved are (i) adsorption, (ii) chelation, or (ii) covalent binding. These methods belong to the following five general categories.
1. Adsorption. The enzymes are adsorbed to several kinds of adsorbents with charged or neutral surfaces. Such materials are used for the separation of proteins by adsorption chromatography. Calcium phosphate gels, carbon, carboxymethylcellulose (CMC), carboxymethyl Sephadex, collagen, silica, gel, titania, alumina, etc. are used. (BSc Industrial Microbiology Notes Study Material)
2. Co-valent binding. Enzyme is bound covalently to a support material using any of the various methods. The enzyme forms a covalent link with active groups of support material.
3. Cross-binding. Enzymes may be cross-linked to a multifunctional one without any solid support. Diazobenzidine, glutaraldehyde, toluene 2, 4-di-iso thiocyanate, and hexamethylene di-isocyanate are some of the reagents used.
4. Entrapment. The enzyme is entrapped inside a cross-linked gel matrix. The gel is allowed to develop in an aqueous solution containing one or more enzymes.
5. Microencapsulation. This is modified entrapment where the enzyme is immobilized within microcapsules prepared from organic polymers.
Industrial applications of immobilized systems
Immobilized systems (enzymes and cells) possess important practical applications in industry. Immobilized systems have been used in antibiotic production. At least in the case of patulin and penicillin G production, the immobilization system can be operated on a continuous basis. Immobilized mycelia of Penicillium chrysogenum are used. (BSc Industrial Microbiology Notes Study Material)
Quite recently immobilised fungal systems have also been applied to environmental problems. Microbial biotechnology has important applications in biodegradation, disposal of wastes, and renewable sources of energy.
BSc Industrial Microbiology Notes Study Material
BSc 2nd Year Sample Model Practice Mock Test Question Answer Papers