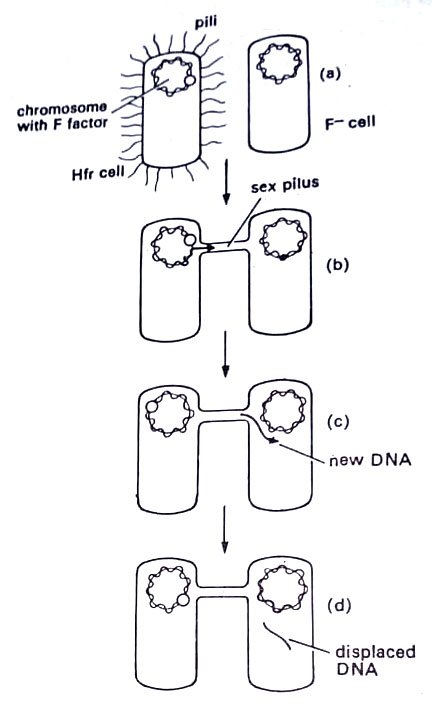
In Hfr strains, the F-factor attaches to the chromosome. This integration is a rare event and requires that an insertion sequence be present on the chromosome to recognize the F-factor. At the point of attachment, the chromosome opens and a copy of one strand is made by the rolling circle mechanism. A portion of single-stranded DNA then passes via the sex plus into the recipient cell. Here it joins the chromosome as in transformation. The first genes to enter the recipient are F-factor genes, but these are not the ones that control the donor state.
Instead, the last segments to enter the recipient are the F-factor genes for the donor state. These rarely enter the recipient, however, because conjugation is often interrupted by such things as broken pili. Thus the F– cell usually remains a recipient, but with some new genes acquired from the donor. In certain cases, the F-factor is indeed transferred to the recipient. When this happens the factor usually detaches from the recipient’s chromosome and enzymes synthesize a strand of complementary DNA. The factor now forms a loop to assume an existence as a plasmid, and the recipient becomes a donor. (BSc Microbiology Microbial Genetics Notes Study Material)
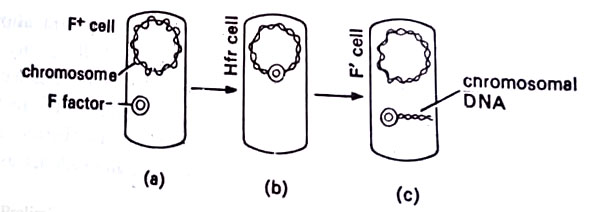
Sexduction. On occasion, the F-factor breaks free from the chromosome of an Hfr cell and resumes an independent status. The Hfr cell then reverts to an F+ cell. Sometimes when the F-factor leaves the chromosome, it takes along a segment of chromosomal DNA. The factor with its extra DNA is now called an F factor (pronounced F-prime). When the F factor is transferred during a subsequent the recipient acquires chromosomal genes from the donor. (BSc Microbiology Microbial Genetics Notes Study Material)
This process is known as sexduction. It results in a recipient with its own genes for a particular process as well as additional genes from the donor for that same process. In the genetic sense, the recipient is a partially diploid organism since there are two genes for a given function. Conjugation has been demonstrated among various generia of bacteria in contrast to transformation which appears to occur only among cells of the same species. This has great significance in the transfer of antibiotic resistance genes carried on plasmids.
When the plasmids are F-factors, the transfer occurs readily. Moreover, when the genes are attached to transposons, the transposons may “jump” from ordinary plasmids to F-factors, after which transfer may occur, Conjugation may also occur in Gram-positive bacteria such as Streptococcus mutants. Only plasmids especially those carrying genes for antibiotic resistance are involved in this species. Similar observations have been made in Bacteroides and Clostridium species. Till now mechanism of plasmid transfer in Gram-positive bacteria is poorly understood and chromosomal transfer is yet to be demonstrated.
Plasmids. Continuing research in bacterial conjugation revealed that several other genetic traits other than chromosomes were present. The genes for the production of some toxins, for the production of pilus, and for antibiotic resistance were found on DNA fragments in the cytoplasm. The word plasmid was coined for these fragments independent of chromosomes. Plasmids are small circular DNA molecules that replicate in cells independently of the chromosome. Such fragments have been termed by different authors, as sex factors, conjugations, extrachromosomal replicons, and transfer factors. (BSc Microbiology Microbial Genetics Notes Study Material)
For instance, the genes responsible for the transfer of resistance to some antibiotics are called resistance transfer factors. Those responsible for the transfer of the property of producing colicins (antibiotics-type substances lethal to closely related strains or species of the bacteria producing them) are called the colicin factors (Cf). The plasmids normally contain only about 2 percent of the total genetic information and multiply independently of the chromosome. They pass quickly from cell to cell as does the F-factor (fertility factor) and are found to be a critical factor in transferable drug resistance (TDR). (BSc Microbiology Microbial Genetics Notes Study Material)
Thus by genetic recombination, a pathogenic um may acquire the genes (for resistance to a particular drug) from a harmless (non-pathogenic) organism. For instance, 1976 saw the emergence of penicillin-resistant gonorrhea organisms, and multiple resistance in pneumonia cocci were located in 1981. The plasmids are not essential to cell growth and may be lost without any harm to the cell.
Those plasmids that attach themselves to the chromosome are called episomes. Thus, the F-factor in Hfr cells is considered an episome. The study on plasmids was pioneered by Stanford University microbiologist, Stanley Cohen who later utilized them in genetic engineering experiments. Plasmids in fact lie at the core of genetic engineering, which offers the potential to increase the range of antibiotics and to increase the availability of any compound in short supply e.g. insulin.
[III] Transduction
This was discovered in 1952 by Joshua Lederberg and Norton Zinder during their search for evidence of conjugation in Salmonella typhimurium. Recombination by this method requires a virus (bacteriophage) as an agent to carry genes from one bacterium to the next. Bacteriophage interacts with bacterial cells by a

the complex process is shown in Figure. The phage first attaches to a receptor site on the bacterial cell wall, after which nucleic acid passes into the cytoplasm of the host cell. (BSc Microbiology Microbial Genetics Notes Study Material)
At this point, either of two events may occur. In the first alternative, the nucleic acid codes for proteins that will form new virus particles identical to the original phage. Components are drawn from the host cell, and the metabolism of the latter is interrupted to such an extent that after some minutes the cell bursts apart to release new bacteriophages. This is the lytic cycle, from which lysing means to break, and the phage that causes the lytic cycle is called a virulent phage. (BSc Microbiology Microbial Genetics Notes Study Material)
In the second alternative, the phage does not cause the lysis of the bacterium. Instead, the phage nucleic acid codes for a substance called a repressor protein. This protein prevents the virus from directing the production of materials necessary for replication. Rather than forming new virus particles, the DNA may exist as a fragment of DNA in the host cytoplasm in a form called the prophage. It may exist as a fragment of DNA outside the chromosome, essentially as a plasmid, or it may attach itself to the chromosome as the F factor does in Hfr strains.
This nonreplicating phage is called a temperate phage and the DNA fragment is known as a prophage. The bacterium that carries the prophage is said to be lysogenic, and the phenomenon where the bacterium and phage DNA coexist is called lysogeny.
The bacterium may remain lysogenic for many generations during which time the viral DNA replicates together with the bacterium. However, at some point in the future, the phage stops coding the repressor protein, and the lytic cycle will begin.
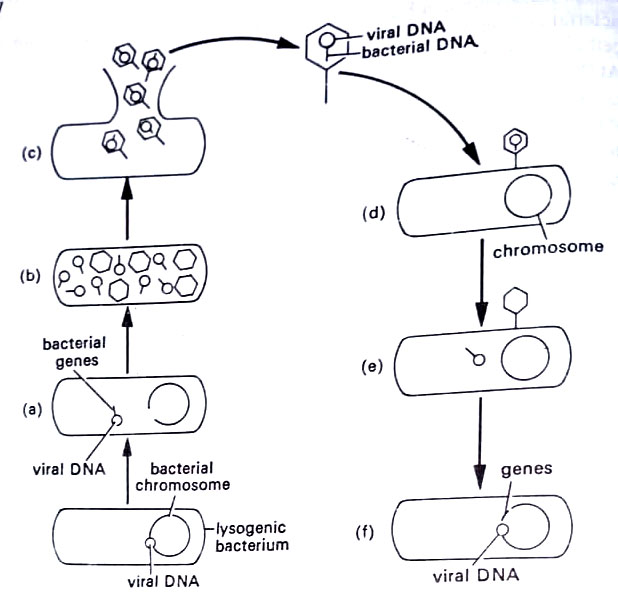
The viral DNA that was attached to the chromosome will now break free and direct the synthesis of those proteins that will yield new viruses. In detaching, however, the viral DNA may carry with it a few bacterial genes from the chromosome. The genes are then replicated along with the viral DNA and they become part of the new phage particles. When the latter is released, copies of the genes are carried along. As the cycle repeats during the next infection, phage DNA enters the new bacterial cells and inserts onto a new chromosome.
However, copies of the original bacterial genes are included, and the bacterium becomes transduced. The bacterial cell now contains its own genes plus several from the original cell. This type of transduction is called specialized transduction, because specific genes are removed from the bacterial chromosome, depending upon where the viral DNA was attached. This occurs in lambda phage. The removal of genes, however, is thought to be an extremely rare event. Another type of transduction, generalized transduction is a more common event.
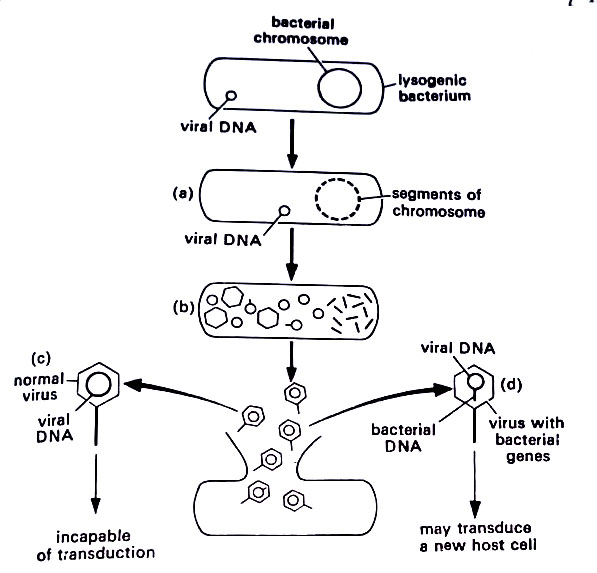
It is mediated by the prophages that have remained in the cytoplasm as plasmids that are not attached to the chromosome. This occurs in Pl phage and many others. The viral DNA lies in the cytoplasm and produces copies of itself for new phage particles. In doing so it may accidentally incorporate small chromosomal segments of bacterial DNA and incorporates into its own DNA.
Some phages may accidentally package only bacterial DNA. In most cases, normal viruses will be liberated from the cell. Occasionally, a virus contains several bacterial genes acquired in the chromosomal segments. If such a virus infects a new cell, whereupon it will attach to the chromosome and transduce the cell as lysogeny is established. In generalized transduction, the viral DNA enters the lytic cycle and forms new virus particles. However, tiny fragments of the bacterial chromosome are sometimes incorporated into the DNA of the new viruses or may occasionally replace the viral DNA. (BSc Microbiology Microbial Genetics Notes Study Material)
This is a random occurrence that may involve any of the bacterial genes, hence the name generalized transduction. Perhaps one phage in a thousand contains bacterial DNA. All bacterial genes are equally available to be picked up by the phage DNA. When the viral particles are released during lysis, the genes are carried along, and on subsequent infection, the genes enter the cytoplasm of the new host cell where they will now function. (BSc Microbiology Microbial Genetics Notes Study Material)
The phenomenon of lysogeny is well-established in modern microbiology. Diphtheria organisms are known to contain bacteriophages that code for the toxin produced during disease. Herpes simplex viruses remain for many years as prophages in the cytoplasm of the body cells, expressing themselves at long intervals. Certain viruses are known to attach to human chromosomes, transforming the cells into tumor cells.
Recombination in Eukaryotes
Unlike prokaryotes, in eukaryotes, the genes are organized into several chromosomes which are present in the nucleus. The number of chromosomes is species-specific and is a stable character of that species. Genetic recombination here is essentially a sexual process involving recombination during meiosis leading to the formation of male and female haploid gametes. The fusion of gametes results in new types of diploids. However, in fungi, there is some complexity. Unlike most eukaryotes, some only exist in the haploid state.
Consider the life cycle of two fungi, Aspergillus nidulans, and Saccharomyces cerevisiae. In A. nidulans, haploid mycelia of different strains fuse and form fruit bodies. In this fruiting body, there is karyogamy and meiosis. During meiosis, the chromosomes from the two nuclei pair up and recombine with each other to give hybrid chromosomes which carry genes from both parent chromosomes. Meiosis is followed by the formation of spores that germinate to produce haploid mycelium. (BSc Microbiology Microbial Genetics Notes Study Material)
However, S. cerevisiae is non-mycelial. The diploid cells formed after fusion can undergo normal growth and division. There are no fruit bodies, and each diploid cell can undergo meiosis to produce haploid cells.
In eukaryotic microbes, there is the alternation of a haploid and a diploid generation. In different forms, there is a wide variation in the relative importance of the haploid and diploid phases for vegetative reproduction and in the degree of sexual differentiation they exhibit. In fungi, the predominant phase may be haploid or diploid or there may be roughly equal roles of both; there may be morphologically distinct male and female forms or there may be sexual conjugation between two similar cells derived from the same clone.
An important characteristic of eukaryotes is the presence of extra-nuclear DNA in organelles such as chloroplasts and mitochondria. Eukaryotic cells may also possess plasmids. S. cerevisiae has a 2µm plasmid that has formed the basis of gene cloning in yeast both in its own right and as a hybrid molecule with bacterial plasmids allowing the analysis of yeast genes both in bacteria and in the yeast itself.
Genetic Engineering
Experimental work on bacterial recombination took a new dimension in the late 1970s when it became possible to insert genes into bacterial DNA and thereby establish a cell line that could produce proteins according to the instructions of microbiologists.
In fact, the real interest in genetic manipulations increased in the 1960s when a group of bacterial enzymes called endonucleases was discovered. These enzymes cleave the phosphate-sugar bonds in the backbone of nucleic acids and could be used to open a bacterial chromosome at the desired point. Moreover, each endonuclease scanned a DNA chain and cleaved it at a restricted point.
For this reason, endonucleases came to be known as restriction enzymes. The existence of these enzymes was first postulated by Werner Arber of the University of Basil (Switzerland) when he noted the bacterial enzymes scissoring viral DNA at selected spots. Hamilton Smith of Johns Hopkins University subsequently isolated a restriction enzyme from a Gram-negative rod, Haemophilus influenza. Daniel Nathans, of Johns Hopkins University, utilized the enzyme in 1971 to split the DNA of the monkey tumor virus, Simian virus40 (SV40). In 1978, the Nobel Prize was awarded to the three scientists. By that time over 100 restriction enzymes had been isolated and characterized.
Among the first scientists to attempt genetic manipulation was Paul Berg of Stanford University. In 1971, Berg and his coworkers opened the DNA molecule of SV40 and spliced it into a bacterial chromosome. In doing so they constructed the first recombinant DNA molecule.
However, the process was very tedious because the bacterial and viral DNAs had blunt ends. Berg used much chemistry to form staggered ends. He later shared the 1980 Nobel Prize in Chemistry with Frederick Sanger. While Berg was performing his experiments in 1971, an important breakthrough came from Herbert Boyer and co-workers at Univ. of California, San Francisco.
The U.S.A. Boyer isolated a restriction enzyme that would nick a chromosome cleanly and leave it with mortise-like staggered ends. The bits of single-stranded DNA extending out of the chromosome easily attached to a new fragment of DNA in recombinant experiments.
Scientists quickly dubbed the single-stranded extensions “sticky ends”. During the same period, Stanley Cohen at Stanford University was working with plasmids of E. coli. He found that he could isolate plasmids easily from the cell and then insert them into fresh bacteria by suspending the organisms in calcium chloride and heating them suddenly to achieve transformation.
Once inside E.coli cells the plasmids multiplied independently and produced copies of themselves in succeeding generations. Cohen’s data thus indicated that it was now unnecessary to work with the larger, less-manageable chromosome. One may well work with plasmids.
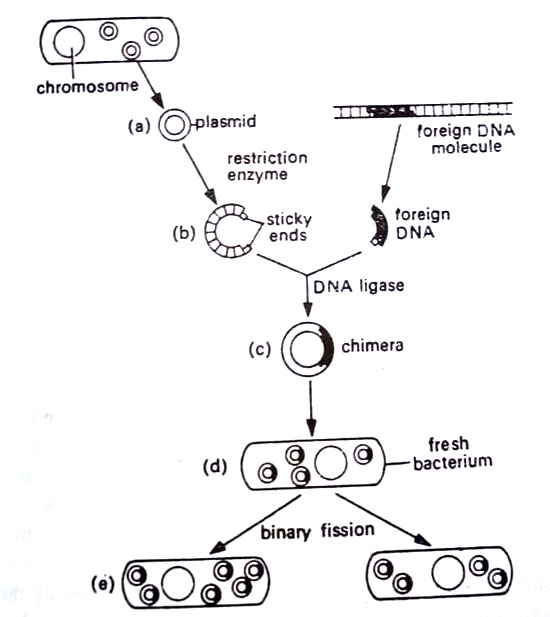
The final link to the process was provided by the DNA ligases. These enzymes are known since the 1960s to function in the replication of DNA and the repair of broken DNA molecules. Essentially they operate in a manner opposite to the endonucleases, that is, they seal together DNA fragments. Working together Boyer and Cohen isolated plasmids from E. coli and opened them with restriction enzymes. Then they inserted a segment of foreign DNA using DNA ligase. Then they implanted the plasmids into fresh E. coli. By 1973, Boyer and Cohen had successfully spliced genes from Staphylococcus aureus into E. coli. The recombined plasmids were called chimeras.
Paul Berg in 1973 proposed to splice SV40 genes to E. coli using the techniques of Boyer and Cohen. Berg was using the SV40 virus because it contained only seven genes and was therefore easy to work with. Other scientists, learning of the proposal, reminded Berg that if the recombined E. coli escaped from the laboratory, it might settle in the human intestine and carry along the tumor genes of the SV40 virus. Berg canceled the idea, and with ten other scientists warned of the potential dangers of recombinant DNA technology.
In 1974, scientists called a moratorium on all such experiments However, scientists since then review the consequences of such techniques. Only the riskiest ones remain under regulation. In 1985, one such experiment, involving the release of bacteria into the environment stirred much controversy. Pseudomonas syringae is present in the phylloplane of many plants. At temperatures below 32°F, it produces a protein that induces ice crystal formation thus causing frost injury to potato plants. Steven Lindow and Nickolas Panapoulas of the Univ. of California 1983 engineered the bacterium that now lacked the culprit protein.
Plants were now able to withstand temperatures as low as 23°F because dew can cool to that point before it turns to ice. Plants were protected from frost and their growing season was also extended. The spray was banned by the court in 1984. However, permission was granted in 1985 by the court. (BSc Microbiology Microbial Genetics Notes Study Material)
Gene cloning procedure
Genetic engineering rests on two of the major discoveries of the last 20 years, namely, plasmids and restriction enzymes. Plasmids have already been described. We have already indicated restriction enzymes also. They are in fact one of the bacterial defense systems against the entry of foreign DNA. The restriction enzyme recognizes a specific sequence of DNA bases and cuts the DNA at or close to that site.
This specific sequence of bases occurs within the DNA of the bacterium but is protected from the action of the restriction enzyme by modification of one of the bases within the sequence. Foreign DNA is not similarly protected so when it enters the cell it is rapidly degraded by the restriction enzyme. (BSc Microbiology Microbial Genetics Notes Study Material)
The plasmids used in genetic engineering are relatives of the naturally occurring plasmids found in microorganisms. Generally, they have been mutated and tailored with enzymes in order to have specific desired properties. Through genetic engineering (as an advantage over conventional genetic techniques) one can transfer a single specific gene between organisms. The steps are:
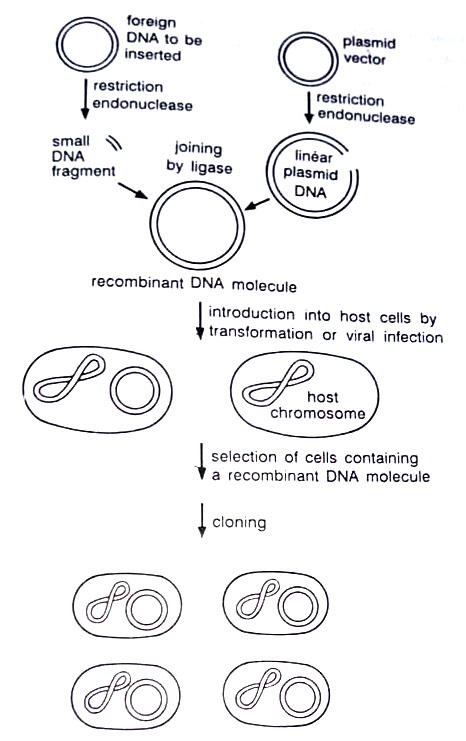
(1) The donor DNA carrying the gene of interest is cut with a restriction enzyme to yield fragments of various sizes, one of which bears the desired gene. A suitable plasmid cut with the same enzyme is mixed with the donor DNA and the two are joined by an enzyme, DNA ligase, to give a series of hybrid plasmids.
(2) The hybrid plasmid DNA is used to transform the host cell and the cells are plated out onto agar. The ratio of the amount of DNA to cells is adjusted in such a way that each cell takes up only one DNA molecule. Thus each colony that grows represents a different piece of the DNA donor carried on a plasmid. One of these carries the gene of interest and can be recognized by the characteristics it confers on the cell.
This gene is then said to have been cloned. This is the basis of genetic engineering. This method can be applied to DNA from any organism and the host can be a bacterium, yeast, other fungus, or plant or animal cell. Genetic engineering has made significant contributions to industrial microbiology and to understanding the possible mechanisms of adaptability in microorganisms.
In brief gene cloning involves (i) isolation and fragmentation of the source DNA and incorporation of the fragments obtained into a cloning vector (segment of DNA used for replication of foreign DNA fragments), with the use of restriction endonucleases to cut and ligase to rejoin DNA molecules (ii) incorporation of the genetically transformed DNA into a recipient organism that can replicate the cloning vector, (iii) detection of the newly transformed cells containing DNA and isolation of a pure culture therefrom; and (iv) growth of a culture of cells containing the cloned DNA fragment. A cloning vector must be able to replicate autonomously in a suitable host.
Plasmids are used as carriers of unrelated DNA for genetic engineering, and the enzymes used for splicing foreign DNA into the plasmid carriers are those used in normal recombination and replication of DNA.
However, at the same time, there are dangers also associated with this technology. There are possibilities of creating new and uncontrollable pathogens from tame microbes like E. coli and yeast. (BSc Microbiology Microbial Genetics Notes Study Material)
Modern Applications of Genetic Engineering
Genetic engineering has wide applications in modern biotechnology. For various industrial processes, this technique may be used in microorganisms as well as with higher organisms. The principle involved is the construction of plasmids of desired biochemical characteristics. Plasmid technology is being hailed by many as the beginning of modern industrial microbiology.
The plasmids are tiny ringlets of DNA, apart from the chromosome, that may contain 2-250 genes. They exist autonomously in the cell. The plasmids can be spliced with genes from an unrelated organism. The genes now function to produce the protein (of the unrelated organisms) in the cell of the host microorganism. The following are the chief possible applications:
(1) Plasmids of one bacterium may be spliced with genes from another bacterium. For example, it is found that plasmids of Pseudomonas will function in other Gram-negative bacteria such as Escherichia, Proteus, or Rhizobium, and that staphylococcal plasmids can be transferred to Bacillus subtilis cells, where they will replicate and express themselves.
(2) Since microbial cells have a much higher metabolic rate, genes of desired enzymes (of commercial values) could be introduced into the plasmids of bacteria. For instance genes of amylase, synthesis could be derived from yeasts by introducing plasmid genes for amylase production. This would enhance the process of beer fermentation. Similarly, genes for cellulase synthesis could be incorporated into plasmids of microbes. The resulting large-scale cellulases could be utilized for cellulose degradation.
(3) Even nitrogen fertilizers may be eliminated by incorporating plasmids, containing bacterial genes for nitrogen fixation, into the plant cells.
(4) Plasmid technology has shown that products like insulin, interferon, vaccines, and human growth hormones may be industrially possible. By 1984, over 200 companies the world over had established gene-splicing experiments and working on industrial applications of genetic engineering. One company in 1980 could harvest insulin from bacteria whose plasmids had been spliced with DNA for this protein. The DNA was from chromosome number 11 of human cells, thus product was identical to human insulin. Marketed by Eli Lilly Corporation, the bacterial insulin, humulin, is identical to human insulin.
In 1980, interferon was produced by genetic engineering from bacterial cells. By 1986, interferon from engineered bacteria was being tested on rabies victims, common cold patients, and cancer patients. Scientists have also inserted genes into bacteria for the production of human growth hormones. This hormone is used to treat dwarfism. In 1986, the hormone became commercially available as protropin.
In June 1981, a vaccine for foot-and-mouth disease was developed by a genetic engineering firm. There are many other products derived from genetic engineering. Urokinase, a clot-dissolving enzyme is produced from genetically engineered bacteria. Endorphin, a painkiller is also derived from bacteria. Bacteria have also been engineered to live solely on toxic wastes in the environment. A gene for the hair-digesting enzyme is inserted into the plasmid of bacteria. (BSc Microbiology Microbial Genetics Notes Study Material)
There are also attempts to engineer plants with bacterial genes that trap N2 and convert it to a form that could be easily taken by the plant. Yeasts are being engineered to yield enzymes for the cheese industry.
BSc Microbiology Microbial Genetics Notes Study Material
BSc 2nd Year Sample Model Practice Mock Test Question Answer Papers