BSc 2nd Year Viruses Caused by Plant Pathogens Notes Study Material
BSc 2nd Year Viruses Caused by Plant Pathogens Notes Study Material: We provide to all the students BSc 1st, 2nd, and 3rd Year notes Study material, question answers, sample papers, mock test papers, and pdf. At gurujistudy.com you can easily get all these study materials and notes for free. Here in this post, we are happy to provide you with BSc 2nd Year Viruses Caused by Plant Pathogens Notes Study Material.

BSc 2nd Year Viruses Caused by Plant Pathogens Notes Study Material
Viruses are too small to be seen with a light microscope. They multiply only in live cells and have the ability to cause disease. They parasitize unicellular plants or animals to large trees and mammals. Viruses attack man and/or animal (causing diseases like influenza, rabies, polio, smallpox, and AIDS) as well as bacteria and mycoplasmas. More than half of all known viruses (a total number of more than a thousand) attack and cause diseases in plants. A single virus can infect different species of plants, and one plant may be attacked by many viruses.
Viruses are remarkable in the sense that they behave as chemical molecules. Each virion consists of nucleic acid and protein, the latter wrapped around the nucleic acid. There is only RNA or DNA, never both in a virus and in most plant viruses, only one kind of protein.
Viruses do not divide and do not produce any reproductive structures as spores. But they multiply by inducing the host cells to form more viruses. They cause disease not by consuming cells or killing them with toxins, but by upsetting the cell metabolism, which in turn leads to the production of abnormal substances by the cell. These are injurious to the life of the plant.
General Characteristics of Plant Viruses
Plant viruses differ greatly from other plant pathogens (fungi, bacteria, etc.) not only in size and shape but also in the simplicity of their chemical and physical structure, methods of infection, multiplication, translocation within the host, dissemination, and symptoms they produce on their hosts.
1. Detection. The mere presence of a virus in the infected cell does not mean that it causes disease. Some viruses may be seen in sections of cells or crude sap from the infected part under an electron microscope. For most plant viruses, it has to be proved that characteristic symptoms are caused by that particular virus (proof of pathogenicity). This job requires (i) the elimination of every other possible cause of disease, and (ii) transmission of the virus from diseased to healthy plants in a way that would exclude transmission of any other causal agents.
The current methods for the detection of plant viruses involve primarily transmission of the virus from a diseased to a healthy plant by budding, gratis, or by rubbing with plant sap. Certain other methods of transmission, such as dodder insect vectors, are also used to demonstrate the presence of the virus. Out of these, only transmission through plant sap is considered proof of the viral nature of the pathogen. The most authentic proof of the presence of a virus in a plant is provided by microscopy and or serology. We shall describe some later under “Techniques in plant virology”.
2. Morphology. Plant viruses differ in shape and size. Usually, they are elongated (rigid rods or flexuous threads), as rhabdoviruses (bacillus-like), or spherical (isometric or polyhedral). The elongated ones are tobacco mosaic virus, maize dwarf mosaic virus, barley stripe mosaic virus, etc., whereas the spherical forms include cowpea chlorotic mottle virus, potato yellow dwarf virus, wheat mosaic virus, lettuce necrotic yellow virus, etc. Some elongated viruses as TMV and barley stripe mosaic virus measure about 15 X 300 nm and 20 X 130 nm respectively, whereas most elongated viruses are usually 10 to 13 nm wide, and from 480 nm to 2000 nm in length.
Among rhabdoviruses, the potato yellow dwarf virus measures 75 X 380 nm wheat mosaic virus, 65 x 270 nm, and the lettuce necrotic yellow virus 52 x 300 nm. (BSc 2nd Year Viruses Caused by Plant Pathogens Notes Study Material)
Most spherical viruses are polyhedral, ranging in diameter from 17 nm to 60 nm. Most plant viruses consist of more than one component. Tobacco rattle virus has two rods, a long (195 X 25 nm) and a shorter (43-110 X 25 nm), alfalfa mosaic virus consists of five components measuring 58 X 18,54 X 18,42 x 18,30 X 18 and 18 x 18 mm. Most isometric viruses have two or three different components.
The surface of plant viruses consists of a definite number of protein subunits, which are spirally arranged in elongated viruses, and packed on the sides of polyhedral particles of the spherical viruses. In cross-sections, elongated viruses appear as hollow tubes with the protein subunits forming the outer coat and the nucleic acid, also spirally arranged, embedded between the inner ends of two successive spirals of the protein subunits. (BSc 2nd Year Viruses Caused by Plant Pathogens Notes Study Material)
The spherical viruses may or may not be hollow, the visible shell consisting of the protein subunits, with the nucleic acid inside the shell and arranged in a yet unknown manner. The rhabdoviruses, potato yellow dwarf, lettuce necrotic yellow virus, etc. are provided with an outer envelope or membrane-bearing surface projections. Inside the membrane is the nucleocapsid, consisting of helically arranged nucleic acid and associated protein subunits. (BSc 2nd Year Viruses Caused by Plant Pathogens Notes Study Material)
3. Composition and structure. A plant virus consists of at least a nucleic acid (NA) and a protein (P). Some viruses consist of more than one size of nucleic acid and proteins, and some of them contain additional chemical compounds such as lipids, polyamines, or specific enzymes.
The proportions of NA and P vary with each virus, with NA making up 5 to 40% and making up the rest 60 to 95%. Normally, elongated viruses contain higher protein percentages and lower nucleic acid, whereas the reverse is true for spherical viruses. The total weight of nucleoproteins of different viral particles varies from 4.6 million mol. wt. units (bromegrass virus) to 39 million mol. wt. unit (TMV) to 73 million mol. wt. units (tobacco rattle virus). The weight of nucleic acid alone ranges only between 1 and 3 million (1-3 X 106) mol. wt. units per virus particle. (BSc 2nd Year Viruses Caused by Plant Pathogens Notes Study Material)
The protein components are composed of repeating subunits. Amino acid content and sequence vary for different viruses, different strains of the same virus, and even for different proteins of the same particle. The complete sequence of amino acids is known only for proteins of TMV and TYMV (turnip yellow mosaic virus). The protein subunit of TMV consists of 158 amino acids in a constant sequence, whereas that of TYMV has 189 amino acids. (BSc 2nd Year Viruses Caused by Plant Pathogens Notes Study Material)
In TMV, protein subunits are arranged in a helix containing 16 1/3 subunits per turn (49 subunits per three turns). The central hole of the virus particle down the axis has a diam. of 40A, whereas the maximum diam. of the particle is 180 A. Each TMV particle consists of approx. 130 helix turns of protein subunits. The nucleic acid is packed tightly between the helices of protein subunits.
In rhabdoviruses, the helical nucleoproteins are enveloped in a membrane. In polyhedral viruses the protein subunits are tightly packed, producing 20, or some multiple of 20 facets, and form a shell. Within this shell, the nucleic acid is folded or otherwise organized.
The nucleic acid of most plant viruses consists of RNA. Only three plant viruses (cauliflower mosaic, dahlia mosaic, and etched carnation ring) have been shown to contain DNA. Both DNA and RNA are long chainlike molecules consisting of hundreds or, more often, thousands of units-nucleotides. RNA usually exists as single strands, though in some viruses also as double-stranded.
The protein coat provides protection against nucleic acid. This itself has no infectivity, though its presence generally increases the infectivity of nucleic acid. Nucleic acid alone is responsible for the synthesis and assembly of both RNA and protein. Infectivity is strictly the property of nucleic acid which in most plant viruses is RNA. The code for the sequence of amino acids consists of a codons-each codon is a triplet.
Replication of Virus (Infection and Virus Synthesis)
Plant viruses enter cells only through wounds made mechanically or by the vectors, or by deposition into an ovule by an infected pollen grain.
The RNA is freed from the protein coat. It then induces the formation (by the cell) of RNA-polymerases (= RNA-synthetases = RNA-replicates). These, in presence of viral RNA (acting as a template) and nucleotides that compose RNA, produce additional, RNA. The first new RNA produced is not viral but a strand that is a mirror image of the virus, and which, as it is formed, is temporarily connected to the viral strand. Thus, the two form a double-stranded RNA that soon separates to produce the original virus RNA and the mirror image strand,
the latter then serving! as a template for more virus RNA synthesis. The replication of some single-stranded RNA viruses that have parts of their RNA in two or more virus particles, of some rhabdoviruses, and of some double-stranded RNA viruses differ much from this method of replication. As soon as new viral nucleic acid is produced it induces the host cell to produce the protein subunits that will form the coat of the virus. For TMV, whose RNA consists of 6400 nucleotides and its protein of 158 amino acids, only 474 nucleotides are required to code the sequence of amino acids in the protein subunit. (BSc 2nd Year Viruses Caused by Plant Pathogens Notes Study Material)
For viral protein synthesis, the part of the viral RNA coding for the viral protein plays the role of mRNA. The virus utilizes the amino acids, ribosomes, and tRNAs of the host, but it becomes its own mRNA and the protein formed is for exclusive use by the virus as a coat or for other functions.
During virus synthesis, part of its nucleic acid also becomes involved with the synthesis of extra proteins (other than coat). Some of these are enzymes, that may activate or initiate the cell chemical reactions that may affect the cell physiology. After the production of new viral nucleic acid and viral protein subunits, cid appears to organize the protein subunits around it, and the two assembled together to form the complete virus particle, the virion. What are the sites in the host cell where viral RNA and protein are synthesized, and the two components assembled? It is known for some viruses.
For TMV, it is shown that free RNA moves into the nucleus and perhaps the nucleolus where it replicates itself. The new viral RNA is then released into the cytoplasm, where it serves as mRNA, and with the help of ribosomes and tRNAs, it produces the protein subunits. The assembly of virion occurs also in the cytoplasm. In other viruses, all these events occur in the nucleus, from which virions are released into the cytoplasm. (BSc 2nd Year Viruses Caused by Plant Pathogens Notes Study Material)
The first intact virions appear in plant cells approx. 10 hr. after inoculation The virions may exist singly or in groups and may form amorphous or crystalline inclusion bodies within the cell (cytoplasm, nucleus, nucleolus) in which they are produced.
Translocation and distribution in plants
The virus moves from one cell to another, and while moving it multiplies in most cells. The viruses move through the plasmodesmata connecting adjacent cells. They infect and multiply in parenchyma cells, and then invade continuously and directly cell-to-cell.
In leaf parenchyma cells the virus moves approx. 1 mm or 8 to 10 cells per day. Moreover, besides this cell-to-cell movement, many viruses are rapidly transported over long distances through the phloem, in the sieve tubes moving as rapidly as 15 cm in the first 6 min. Once within the phloem, the virus moves rapidly in the phloem toward apical meristems or other regions such as tubers, and rhizomes.
The distribution of viruses within plants varies with the virus and the plant. There may be localized distribution to one area of a part or one part of the plant, resulting in local lesions development. In other cases, there may be systemic development, when they involve all live cells of the plant.
Symptoms Caused by Plant Viruses
Viruses cause several kinds of symptoms in diseased plants. The viruses may be systemic, associated with each stage of the life cycle of the plant, or localized, being restricted to some parts of the plant only. In almost all viral diseases occurring in the field, the virus is present throughout the plant (systemic infection) and the symptoms are called systemic symptoms. In many artificially inoculated plants and in some natural infections, viruses form local lesions (local infection).
The most common and sometimes the only kind of symptom is the reduced growth rate of the plant. This results in various degrees of dwarfing or stunting of the entire plant. All viral diseases cause some degree of reduction in total yield.
The most obvious symptoms are usually those appearing on the leaf, but some viruses may cause striking symptoms on the stem, roots, and fruit with or without symptom development on the leaves. Many viruses may infect some hosts without causing the development of visible symptoms. Such viruses are called latent viruses and the hosts are called symptomless carriers. In some cases, there develop symptoms, with some viruses remaining temporarily symptomless under certain environmental conditions (high/low temp.). Such symptoms are called masked. Finally, plants may show acute or severe symptoms soon after infection.
The most common types of plant symptoms produced by systemic I infections are mosaics and ringspot. A large number of other fewer com symptoms are a stunt, dwarf, leaf roll, yellows, streak, pox, enation, tumors, the pin of the stem, pitting of fruit, flattening and distortion of the stem, etc. These symptoms are accompanied by other symptoms on other parts of the same plant. Brief notes on the different kinds of symptoms now follow:
- Mosaics. These are characterized by light-green, yellow, or white areas intermingled with the normal green of the leaves or fruit, or of whitish areas intermingled with areas of the normal colour of the flower or fruit. Depending on the intensity or pattern of discolorations, mosaic-type symptoms may be described as mottling, streak, ring pattern, line pattern, vein-clearing, vein banding, vein thickening, chlorotic spotting, etc.
- Mottles. It is a kind of mosaic, where on the leaves there develops an irregular pattern of indistinct light and dark areas. Like mosaics, there are green and white or green and yellow areas. (BSc Viruses Caused by Plant Pathogens Notes & Study Material)
- Yellows (chlorosis). In extreme cases of mosaics and mottles, the leaf may become almost completely yellow due to chlorosis.
- Vein-clearing. In this case, there is chlorosis of leaf tissue in close proximity to the veins. The tissue close to the veins turns yellow, the remaining area appears green. This is very common in bhindi. (BSc Viruses Caused by Plant Pathogens Notes & Study Material)
- Vein-banding. Here the parenchyma close to the veins is green and the rest of the lamina surface shows chlorosis i.e. becomes yellow.
- Ring spots. These are characterized by the appearance of chlorotic or necrotic rings on leaves and sometimes also on fruit and stem.
- Enations. These are small outgrowths on the leaf, stem, etc. This is usually associated with mosaics. Ex. tobacco enations.
- Leaf-curling or leaf-rolling. These are common in papaya, tomato, potato, etc., where leaves become curled and rolled to varying extents.
- Fern leaf, shoestring. Here leaf lamina is greatly suppressed.
- Stunting. The general growth of the entire plant is affected resulting in an unusually shorter size of plant.
- Virescence. Here entire flower or petals turn to green colour. It is a type of phyllody.
- Tumors. These are gall-like structures developing on roots or stems. In the Fiji disease of sugarcane, elongated galls on the leaf are formed.
- Witche’s broom. Here leaves become very much reduced, and internodes are also shortened. There is an abnormal growth of leaves turning to a den-packed broom-like structure. (BSc Viruses Caused by Plant Pathogens Notes & Study Material)
- Little leaf. Here the leaves are reduced in size. In the fern leaf, there is much suppression of the lamina.
Transmission of Plant Viruses
Plant viruses rarely come out of the plant spontaneously. Therefore, viruses are not disseminated by wind or water. Even when they are carried in the debris plant sap, they would cause infections only when coming in contact with the contents of the wounded live cell. Viruses are transmitted from plant to plant (i.e. they actually spread) in a number of ways-vegetative propagation, mechanical transmission through the sap, and by seed, pollen, insects, mites, nematodes, fungi dodder, etc.
1. Vegetative propagation. Any part of the plant used for vegetative propagation will transmit the viruses to the progeny. Thus viruses are transmitted by budding, grafting, cuttings, tubers, corms, bulbs, rhizomes, etc. This mode of transmission is most important for ornamental trees and shrubs (propagated by budding, grafting, or cuttings) and the field crops like potatoes and most florist’s crops, which are usually propagated by tubers, corms, or cuttings. (BSc 2nd Year Viruses Caused by Plant Pathogens Notes Study Material)
Particularly in trees, viruses are transmitted through natural root grafts of adjacent plants. For several tree viruses, natural grafts are the only known means of the tree-to-tree spread of the virus. (BSc Viruses Caused by Plant Pathogens Notes & Study Material)
2. Mechanical transmission through the sap. Under natural conditions direct transfer of sap through contact of one plant with another is uncommon. Such mechanical transmission may take place between closely placed plants. Strong wind may cause the leaves of adjacent plants to rub together, and if wounded, some of their sap is exchanged, thus transmitting any virus present in the sap. Potato virus X (PVX) is most easily transmitted in this way.
Plants are wounded by men during cultural practices in the field or greenhouse, and some of the virus-infected sap adhering to the tool tally is transferred to wounded plants. TMV on tobacco and tomato spreads rapidly in this way. Occasionally virus infected sap is transferred from one plant to another on the mouthparts or bodies of animals feeding on and moving among the plants. Mechanical transmission is the only authentic means to prove the ability of infection by a virus.
3. Seed transmission. This is not as common as the abovesaid two methods. More than 100 viruses are reported to be transmitted by seed. However, only a small portion (1-30%) of the seeds derived from virus-infected plants transmit the virus. In some tobacco ringspot virus in soybean, almost 100% of seeds of infected plants transmit the virus. Barley stripe mosaic virus also has high transmission of 50-100% seeds. In most cases, the virus comes primarily from the ovule of infected plants.
4. Pollen transmission. Virus transmitted by pollen not only infects the seed and the seedling growing from it but more importantly, they can also spread through fertilized flower and down into the mother plant, that thus becomes infected with the virus. This method is important in the stone fruit ringspot virus of some plants.
5. Mite transmission. Members of the family Eriophyidae are shown to transmit nine viruses, including wheat streak mosaic, peach mosaic, and fig mosaic viruses. These mites have piercing and sucking mouthparts. Mite transmission appears to be specific since each of the mite species has a restricted host range. In such cases, a particular mite is the only known vector for the virus or viruses transmitted by it. Some of the mite-transmitted viruses are stylet borne, whereas others are circulatory. One mite (Tetranychidae, spider mites) is known to transmit Potato virus Y. (BSc 2nd Year Viruses Caused by Plant Pathogens Notes Study Material)
6. Nematode transmission. About 12 viruses are shown to be transmitted by one or more species of three genera of soil-inhabiting, ectoparasitic nematodes. Members of the genera Longidorus and Xiphinema are vectors of tobacco ringspot, tomato ring spot, raspberry ringspot, cherry leaf roll, tomato black ring, grape fanleaf viruses, etc., whereas those of the genus Trichodorus transmit tobacco rattle and pea early browning viruses. Nematodes feed on the roots of infected plants and then move on to the roots of healthy plants. Larvae as well as adults can acquire the viruses.
7. Fungus transmission. Olpidium, a root-infecting chytrid fungus is known to transmit at least four viruses, tobacco necrosis, cucumber necrosis, lettuce big vein, and tobacco stunt viruses. Four other fungi, Synchytrium, Polymyxa, Spongospora, and Pythium transmit potato virus X, wheat mosaic virus, potato mop-top virus, and beet necrotic yellow vein virus respectively. The viruses are present in or on the zoospores and resting spores. (BSc 2nd Year Viruses Caused by Plant Pathogens Notes Study Material)
8. Dodder transmission. Several viruses can spread through the bridge formed between two plans by twining stems of the parasitic phanerogam, dodder (Cuscuta sp). Many viruses spread in this way between plants of widely different taxonomic species.
9. Insect transmission. The most common and economically important method of transmission of viruses in the field is by insect vectors. The order Homoptera which includes aphids and leafhoppers contains the largest number and the most important insect vectors of plant viruses. Whiteflies, the mealy bugs, scale insects, and treehoppers, belonging to this order also transmit viruses but are not economically as important as aphids and leafhoppers. Other insect vectors are true bugs (Hemiptera), thrips (Thysanoptera), beetles (Coleoptera), and grasshoppers (Orthoptera). (BSc 2nd Year Viruses Caused by Plant Pathogens Notes Study Material)
The most important vectors, i.e. aphids, leafhoppers, and other members of Homoptera, as well as true bugs, have piercing and sucking mouthparts; all other vectors have chewing mouthparts and the transmission by the latter is much less common.
On the basis of the virus-vector relationship (where the virus is carried by a vector, and behaviour of the virus within the vector), the viruses are also categorized as follows:
(a) Non-persistent or stylet-borne viruses. These are the viruses carried by insects (with sucking mouthparts) on their stylets.
(b) Persistent or circulative viruses. These are the viruses accumulated by the insects (with sucking mouthparts) internally and, after their passage through insect tissues, they have introduced again into plants through the mouthparts.
(c) Propagative viruses. These are some of the circulative viruses that may multiply in their vectors.
The viruses transmitted by insects with chewing mouthparts may also be circulative or they may be carried on the mouthparts (i.e, stylet-borne).
Aphids are the most important insect vectors of plant viruses and transmit the great majority of all stylet-borne viruses. More than 200 species are known as vectors of plant viruses. As a rule, several aphid species can transmit the same stylet-borne virus and the same aphid species can transmit several viruses, but in many cases, the vector-virus relationship is quite specific. Aphids generally acquire the stylet-borne virus after a brief feeding period on the diseased plant, for only a few seconds (30 seconds or less), and can transmit the virus after transfer to and feeding on a healthy plant for a similarly brief period.
After the acquisition, the aphids remain viruliferous from a few minutes to several hours, after which they can no longer transmit the virus. In some cases of aphid transmission of circulative viruses, aphids can not transmit the virus immediately but must wait several hours ager feeding. However, once they start to transmit the virus, they continue to do so for many days. The viruses in stylet-borne viruses are borne on the tips of the stylets. Several species of leafhoppers are involved in the transmission of a number of plant viruses.
Leafhopper-transmitted viruses cause disturbance primarily in the phloem region. All leafhopper-transmitted viruses are circulative and several are known to multiply in the vector ie. are propagative. Most leafhoppers require a feeding period (one to several days) before they become viruliferous, but once they acquire the virus they remain viruliferous for the rest of their lives.
Control of Plant Viruses
Methods to control viral diseases are generally similar to those used for other pathogens, except that as yet chemicals find little application in virus control, although they may be used for vectors (insecticides). Moreover, vectors involved in viral diseases have also complicated the problem in their control. The following methods are used in their control of viral diseases of plants:
1. Exclusion. The best way to control a virus disease is to keep it out of the area through quarantine, inspection, and certification systems. The existence of symptomless hosts and the absence of obvious symptoms in seeds, tubers, bulbs, and nursery stock make quarantines ineffective. Eradication of all sources of inoculum can be effective in annual crops. Diseased plants should be destructed which would eliminate the inoculum from the field. (BSc 2nd Year Viruses Caused by Plant Pathogens Notes Study Material)
2. Control of vectors. Plants may be protected against some viruses by protecting them against vectors. Controlling the insect vectors and removing the weeds which serve as hosts (for vectors) may help in controlling the disease.
3. Use of virus-free planting material. The use of the virus-free seed, tubers, budwood, cuttings, etc. is the single most important method for the control of viruses, especially those lacking insect vectors. Periodic indexing of mother plants producing such planting material is necessary. Several types of inspection and certification programmes are now in effect in different areas producing seeds, tubers, and nursery stock for vegetative propagation. (BSc 2nd Year Viruses Caused by Plant Pathogens Notes Study Material)
4. Resistant varieties. If adequate resistance genes are found, this is the most satisfactory method. Many plant varieties are resistant to certain viruses been developed. (BSc 2nd Year Viruses Caused by Plant Pathogens Notes Study Material)
5. Physical methods. Viruses can be inactivated by heat. Dormant, propagative parts are dipped in hot water (35 to 54°C) for a few minutes to an hour whereas actively growing plants are kept in greenhouses or growth chambers at 35 to 40°C for several days, weeks, or months.
6. Tissue culture. Virus-free plants can also be produced from virus-infected plants by the culture of shoot apical meristems (0.1 mm to 1 cm).
7. Modification of cultural practices. Agricultural and horticultural practices may be modified to reduce viral attacks, sometimes through their effect on vectors. The sowing date may be adjusted; other practices including reduction of close spacing, mixed cropping pattern (susceptible and non-susceptible plant species), etc, are effective.
8. Chemical methods. So no viricides are available. Foliar application of some growth regulators such as gibberellic acid has been effective in stimulating the growth of virus-suppressed axillary buds in sour cherry yellows. A similar spray with gibberellic acid can overcome the stunting induced by etch virus on tobacco. (BSc 2nd Year Viruses Caused by Plant Pathogens Notes Study Material)
Techniques in Plant Virology
As indicated earlier, one has to prove the pathogenicity of the virus. For this, the virus particles are to be isolated from the plant, purified, and then inoculated into healthy plants for studying the development of the symptoms.
There are several methods that are used for isolating the virus and purifying it. The study of symptom development is made through the mechanical (sap) transmission method. The following are the methods used.
1. Purification of the virus. Isolation or as it is usually called, purification of viruses is done through a series of steps. These steps are shown in Figure and are as follows:
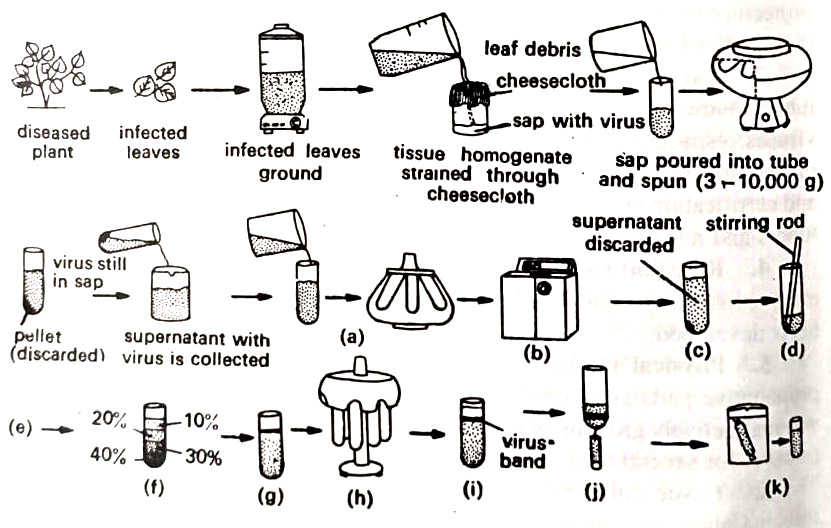
(a) Selection of a suitable buffer System. Since viruses are susceptible to small changes in pH, ionic strength, and the type of ions, the selection of a suitable buffer is very important since viruses are also susceptible to oxidation, a reducing agent, as ascorbic acid or Na2SO3 is also included in the solution used during grinding.
(b) Grinding of infected tissues. Infected tissues could be ground, chopped, homogenized, squeezed, or squashed by hand or motor-operated devices. After the disintegration of the tissue, the juice is passed through several layers of cheesecloth to remove large particles. The crude juice now contains plant cell organelles, dissolved salts, and sugars including viruses.
(c) Removal of extra particles and solutes. The crude juice is treated in various ways and sedimented by centrifugation. This part is also called clarification. The supernatant liquid above the pellet in the centrifuge tube still contains the virus. Sometimes in addition to centrifugation, the crude juice is filtered through any of the filter acids, or the virus is precipitated by organic solvents. With this step, most of the host contaminants are removed. (BSc 2nd Year Viruses Caused by Plant Pathogens Notes Study Material)
(d) Further purification. The actual separation of the virus particles from other macromolecules is done by several methods, that also remove the solutes. The methods are gel filtration, chromatography on Sephadex, sepharose or various agar gels, electrophoresis, and ultra-centrifugation.
Of these, the most commonly used technique is ultracentrifugation of the plant sap. This involves three to five cycles of alternate high (40,000 to 100,000g or more) and low (3000 to 10,000 g) speeds. This concentrates the virus and separates it from host cell contaminants.
Several modifications of the ultra-centrifugation technique, particularly density gradient centrifugation (adapted by M. Brakke of USDA at Lincoln, U.S.A.) are presently employed with excellent results. Sucrose solutions of decreasing concentrations from 60 % – 5% are layered one on top of another in a plastic centrifuge tube, and allowed to spin in a nearly horizontal position. The virus band in the gradient can be clearly seen. The band is collected by inserting a syringe into the plastic centrifuge tube.
(e) Concentration and storing of the viral fraction. Once the virus is obtained in a relatively pure state, it can be concentrated by (i) removal of most of the solvent from the suspension, or (ii) removal of the virus from the suspension, Absorption of water by dry gels, ultrafiltration and dialysis are common methods to remove the solvent from the virus suspension, ‘whereas ultracentrifugation, electrophoresis, crystallization. chromatography and microfiltration are the methods to remove viruses from the suspension.
The purified viral particles obtained are then used for infection, electron microscopy, and serology.
2. Infection studies. (Mechanical or sap transmission). The purified viral particles are then mechanically deposited into a healthy host to observe the symptom development. For routine studies, the crude or partially purified sap is prepared from infected parts of the plant (young leaves, floral petals), etc. These infected parts are ground with a mortar and pestle or with some other grinder. A buffer solution (usually phosphate buffer) may be added for the stabilization of the virus.
Expressed sap is strained through cheesecloth and centrifuged at low speeds (to remove tissue fragments) or at alternate low and high speeds (if the more purified virus is needed). The crude or partially purified sap is then applied to the leaf of young plants, previously dusted with an abrasive such as 600-mesh carborundum added to aid in the wounding of the cells. The sap is applied by gently rubbing the leaves with cheesecloth or a gauze pad dipped in the sap with a finger, a glass spatula, a brush, or a small sprayer. Inoculated plants are kept in the greenhouse and observed for the development of symptoms (2-21 days).
3. Serology of viruses. Serology has been useful in identification of plant viruses as well as in their control. The purified virus is injected into a mammal (rabbit, mouse, horse) or bird (chicken). The antibodies thus produced and the virus (to be identified) are brought together in many ways. The most common method is the precipitin reaction. They are mixed in solution, or they meet at the interface between two solutions, or they diffuse towards each other through an agar gel and meet in a zone in suitable concentrations (Quchterlony test).
In all these reactions, there appears either a precipitate at bottom of a test tube or a band at the interface where the two meet (antigen, the virus, and the antibody). (BSc 2nd Year Viruses Caused by Plant Pathogens Notes Study Material)
Tobacco Mosaic Disease
This is the best known of all virus diseases of plants, and worldwide in distribution. This disease affects more than 150 genera of primarily herbaceous. dicotyledonous plants including many vegetables (potato, tomato, cucurbits), flowers, and weeds. There are serious losses in yield as well as the quality of tobacco, tomato, and some other crop plants. It is symptomless on apples and grapes. TMV affects plants by damaging leaves, flowers, and fruit and causes stunting of the plant.
Symptoms
The symptoms include various degrees of chlorosis, curling, mottling, dwarfing, distortion, blistering dwarfing, distortion, and discoloration of flowers, and in some plants even the development of necrotic areas on the leaf.
The most common symptom of tobacco is the appearance of mottled dark-green and light-green areas on leaves. The dark green areas are thicker light green areas. (BSc 2nd Year Viruses Caused by Plant Pathogens Notes Study Material)
Stunting of young plants is common and is accompanied by slight downward curling and distortion of leaves, which may become narrow and elongated rather than normal oval shape. The petioles may become enlarged (puckered) with enlarged capitate hairs. Old leaves may not show symptoms, young ones develop typical symptoms. (BSc 2nd Year Viruses Caused by Plant Pathogens Notes Study Material)
Causal organism
Tobacco Mosaic Virus (TMV) is rod-shaped, 300 nm long by 15 nm in diameter. Protein (P) consists of approximately 2130 subunits and each subunit consists of 158 amino acids. The protein subunits are arranged in a helix.
The nucleic acid (NA) is single-stranded RNA and consists of about 6400 nucleotides. The RNA strand also forms a helix parallel with that of protein and is located on the protein subunits and approx. 208 out from the inner end of the protein subunits. The mol. Wt. of each virus particle is between 39 and 40 million mol. wt. units. (BSc 2nd Year Viruses Caused by Plant Pathogens Notes Study Material)
TMV is one of the most thermostable viruses known, the thermal inactivation point of the virus in undiluted plant juice being 93C. However, in dried infected leaves, the virus retains infectivity even when heated at 120°C for 30 mts. The infected plant may contain up to 4g of virus per litre of plant juice and the virus retains infectivity even at dilutions of 1: 1,000,000.
In ordinary plant sap, the virus is inactivated in 4-6 weeks, whereas in sterile bacteria-free sap the virus may survive for 5 years, and in TMV-infected leaves kept dry in the laboratory the virus remains infectious for more than 50 years.
TMV is transmitted readily through mechanical sap, grafting, and dodder. It is not transmitted by insects, except occasionally through their contaminated feet and jaw. The most common method of transmission of TMV in fields and greenhouses is through the hands of workers handling infected and healthy plants. (BSc 2nd Year Viruses Caused by Plant Pathogens Notes Study Material)
Disease development
TMV survives in infected leaves and stalks in the soil, on surfaces of contaminated seeds, on contaminated seedbed cloth, and in natural leaf and manufactured tobacco including cigarettes, cigars, etc. The virus initially infects wounded tissues of tobacco seedlings in seedbeds or of transplants in the field. (BSc 2nd Year Viruses Caused by Plant Pathogens Notes Study Material)
Then it spreads in the field throughout the season. TMV in all plants produces systemic infections, invading all parenchyma cells of plants. The virus moves from cell to cell through the phloem. In the cytoplasm of the cell, TMV appears as crystalline aggregates and as amorphous bodies (x-bodies). (BSc 2nd Year Viruses Caused by Plant Pathogens Notes Study Material)
Control
(1) Sanitation is the main method. The crop should not be grown at least for two years in seedbeds or fields where the diseased crop was grown. Removal of diseased plants and of some solanaceous weeds harboring the virus early in the season helps in the reduction and elimination of the subsequent spread of the virus. (BSc 2nd Year Viruses Caused by Plant Pathogens Notes Study Material)
(2) Chewing and smoking of tobacco during handling of tobacco and other susceptible plants should be avoided.
(3) Workers in the field must wash their hands with 3% trisodium phosphate or soap.
(4) Equipment and instruments used in plantations must be sterilized.
(5) TMV-resistant varieties of tobacco must be grown, though these may be of low quality.
Yellow Vein Mosaic of Bhindi
This disease of bhindi (Okra) has taken a severe form in our country. Infection of four to five-week-old plants results in unusual retarded growth and only a few leaves and fruits are formed on such plants. Such plants result in about 94% loss of crop yield. The damage is relatively less on old plants. (BSc 2nd Year Viruses Caused by Plant Pathogens Notes Study Material)
Symptoms
The chief symptoms develop on leaves. There occurs vein learing and veinal chlorosis of leaves. Infected leaves exhibit a very distinct yellow network of veins along with thickened veins and veinlets. In severe cases, the chlorosis may involve interveinal areas resulting in the yellowing of the entire leaf. Fun dwarfed malformed, distorted, and yellow-green in colour.
Causal organism
The Yellow Vein Mosaic Virus (YVMV) of bhindi is not transmitted mechanically through the sap. It may artificially be transmitted through grafting. In the field, YVMV is transmitted by whitefly Bremisia tabaci, and perhaps also by bhindi leafhopper, Empoasca devastans. There are several weed hosts of this virus. These are Croton sparsiflora, Malvastrum tricuspidatum, and Ageratum sp., growing along the roadside and in wasteland areas. (BSc 2nd Year Viruses Caused by Plant Pathogens Notes Study Material)
Control
(1) Protection of the crop from white flies and other insects, by spraying with Follidol (0.3%) or other insecticides. The spray must be done early, just after the seedlings come out, say within three weeks after germination.
(2) Four to six sprays of systemic insecticides, ekatox, metasystox, rogor, etc.
(3) One or two applications of thimet or disyston granules to soil.
(4) Eradication of weed hosts.
(5) Disease-resistant cultivars. Some wild species of Hibiscus and Abelmoschus have been found good sources of resistance.
Leaf Mosaic of Cucurbits
The leaf mosaic virus disease affects a number of cucurbits in almost every area where they are grown. This disease is very common in several cultivated cucurbits including fruit and vegetable crops. It has a wider host range.
One of the viruses on cucurbits for example is the cucumber mosaic, which attacks a greater variety of vegetables, ornamentals, and other plants. Among the most important vegetables and ornamentals attacked by this virus are cucumbers, melons, squash, peppers, spinach, tomato, celery, beets, banana, gladiolus, petunias, etc. (BSc 2nd Year Viruses Caused by Plant Pathogens Notes Study Material)
Symptoms
Young seedlings are never attacked in the field during the first few weeks. Generally 5-6 week old plants are attacked. Young leaves become mottled, distorted, and wrinkled, and their edges begin to curl downward. Plants become dwarfed, and they produce few flowers and fruits. The plants develop a bushy appearance with leaves forming a rosettelike clump near the ground. Fruits develop pale green or white areas intermingled with dark green, raised areas. (BSc 2nd Year Viruses Caused by Plant Pathogens Notes Study Material)
Causal organism
The virus is polyhedral, 30 nm. diam. The virus consists of 180 protein subunits, single-stranded RNA, and a hollow core. The mol. wt. is 5.8-6.7 million of which 18% is RNA and the rest 82% protein. The thermal inactivation point is about 70°C, while the dilution endpoint is about 1: 10,000. There are many strains of cucumber mosaic virus alone. (BSc Viruses Caused by Plant Pathogens Notes & Study Material)
The virus is readily transmitted by sap and also by many aphids. The virus survives in many perennial weeds, flowers, and crop plants. Insect vectors and cultivation and handling of plants, especially at picking time spread the virus in the field to healthy plants. Sometimes the whole field of cucurbits begins to turn yellow with mosaic, immediately after the first pick. The virus produces systemic infection in cucurbits and most other host plants. (BSc 2nd Year Viruses Caused by Plant Pathogens Notes Study Material)
Control
(1) Elimination of weed hosts.
(2) Control of insect vectors.
(3) Resistant varieties.
Potato Spindle Tuber
The disease is caused by a viroid and causes quite severe losses. In some regions, it is one of the most destructive diseases of potatoes. It attacks all varieties and spreads rapidly. Though this disease also attacks tomatoes but is of little economic value in this crop.
Symptoms
Infected potato plants become erect, spindle-shaped, and dwarfed. The leaves are small and erect and the leaflets are darker green, occasionally being rolled and twisted. The tubers are elongated with cylindrical middle and tapering ends. The tubers are smoother, with more numerous, conspicuous, and shallower eyes. Yield is reduced to about 25% or more.
Causal organism
The Potato Spindle Tuber Viroid (PSTV) is the first recognized viroid. PSTV is an infectious RNA of low mol. wt., approx 80,000 daltons. RNA is a single-stranded molecule with extensive regions of base pairing.
Under an electron microscope, purified denatured PSTV appears as short strands about 50 nm long with a thickness of a double-stranded DNA. Sap from the infected plant is infective even at dilutions of 1: 1000 to 1: 10,000. After heating at 75 to 80°C for 10mts. PSTV is quickly inactivated in expressed sap of an infected plant. (BSc 2nd Year Viruses Caused by Plant Pathogens Notes Study Material)
However, infectivity remains intact in phenol-treated sap. PSTV is mechanically transmissible. It spreads mainly by knives used to cut healthy and infected potato “seed” tubers and during handling and planting of the crop. This viroid is also transmitted by pollen and seed and by several insects including some aphids, grasshoppers, flea beetles, and bugs. (BSc Viruses as Plant Pathogens Notes Study Material)
The viroid infects and then spreads systemically throughout the plant. Little or nothing is known about the mechanism of spread within the plant, and the development of symptoms. (BSc 2nd Year Viruses Caused by Plant Pathogens Notes Study Material)
Control
Planting of only PSTV-free potato tubers in the field.
BSc 2nd Year Viruses Caused by Plant Pathogens Notes Study Material
BSc 2nd Year Sample Model Practice Mock Test Question Answer Papers