BSc 2nd Year Biotechnology and Plant Pathology Notes Study Material
BSc 2nd Year Biotechnology and Plant Pathology Notes Study Material: BSc is a three-year program in most universities. Some of the universities also offer BSc Honours. Out of those, there are BSc 2nd Year Study Material, BSc Sample Model Practice Mock Question Answer Papers & BSc Previous Year Papers. At gurujistudy.com you can easily get all these study materials and notes for free. Here in this post, we are happy to provide you with BSc 2nd Year Biotechnology and Plant Pathology Notes Study Material.

BSc 2nd Year Biotechnology and Plant Pathology Notes Study Material
Recent new technologies such as tissue culture and genetic engineering could be utilized in science as well as the art of plant pathology. Plant biotechnology helped not only in the management of plant pests and pathogens, including the development of disease-resistant cultivars but could also add to our understanding of the precise mechanisms of pathogenesis and host resistance. New technologies could contribute to environmental microbiology and plant pathology and some of the ways and areas even overlap.
Biotechnology impinges upon plant pathology in several ways. The most obvious of these ways are considered briefly in the following pages. It would be evident from the account that tissue culture technology contributed to the increased production of plants through rapid clonal propagation to obtain pathogen, free mother plants. Callus and single-cell cultures are used to study the behavior of pathogens. (BSc 2nd Year Biotechnology and Plant Pathology Notes Study Material)
Plant protoplast technology is used for (i) viral replication and physiological changes in the host cell. (ii) introducing disease resistance genes, (iii) selection of desired (disease-resistant) protoplasts, developed as a result of somaclonal variation for subsequent propagation, (iv) evaluation of antimicrobial chemicals, and (v) transmission of resistance genes into sexually incompatible hosts through protoplast fusion. Haploid plant protoplasts obtained from different sources are also used for several studies in plant pathology.
Using suitable vectors, desired genes from suitable sources (as resistance genes) are cloned and then introduced into the host plants. If able to express, disease-resistant varieties can be developed through recombinant DNA technology. These aspects have been considered briefly below.
Tissue Culture Techniques of Importance to Plant Pathology
Though all tissue culture techniques have some relevance to plant pathology, some most important ones are as follows.
[I] Rapid clonal propagation
Explants taken from suitable parts of the plant, including leaves also in some cases, are used. Apical meristems, 0.4-0.8 mm. long are used to obtain pathogen-free plants. (BSc 2nd Year Biotechnology and Plant Pathology Notes Study Material)
[Il] Callus and single cell culture
A large number of identical plants are produced in culture by this method. Cultures of callus, single cells, and the plantlets regenerated from them are used to study the behavior of pathogens, particularly rusts, powdery mildews and downy mildew fungi, many viruses as well as bacteria. The effect of toxins and other chemicals secreted by the pathogen, on the host physiology is also studied by this technique. (BSc 2nd Year Biotechnology and Plant Pathology Notes Study Material)
(III) Plant protoplasts
The most common uses of protoplast in plant pathology are as follows.
1. Study of virus replication and physiological changes in the host cell. Protoplasts are inoculated with the virus in presence of usages, such as poly L ornithine or polyethylene glycol. The mixture is incubated at room temperature for 10-20 min, and the protoplasts are then washed with mannitol or nutrient solution, or both. Virus replication rate is monitored by use of suitable methodology (electron microscopy, bioassay, ELISA fest, etc).
2. Protoplast inoculation with genetically- engineered vectors of genetic material. Two successful vectors used for the introduction of foreign DNA into plant cells include (i) the bacterium, Agrobacterium tumefaciens or its modified Ti plasmid and (ii) the double-stranded DNA virus, cauliflower mosaic virus. Protoplast inoculation has been a very useful technique in plant pathology. This is the most likely method for introducing disease-resistance genes into important agronomic plants. (BSc 2nd Year Biotechnology and Plant Pathology Notes Study Material)
3. Selection of protoplasts and of protoplasts-derived plants resistant to infection, toxins, or other toxic substances. Plants developing due to somaclonal variation (regenerated from callus, single cells, or protoplasts) are studied for their reaction to infection, toxin effect, etc. The protoplasts and protoplast-derived plants that survive the infection or toxin treatment etc are then selected for further propagation, study, and incorporation into the breeding programmes.
4. To evaluate antiviral compounds. Virus-infected protoplasts are treated with different antiviral chemicals. These are suitably monitored and tested by bioassay, ELISA, etc. (BSc 2nd Year Biotechnology and Plant Pathology Notes Study Material)
5. Protoplast fusion for transmission of resistance genes into the sexually incompatible hosts. Protoplasts are mixed using a suitable usage. Resultant somatic hybrids in which the additional genome contains effective genes of resistance are important to us.
[IV] Haploids
Haploids of many species can be produced by another culture of suitable developmental stage. Haploid plants or protoplasts obtained from haploid callus, plants, or directly from pollen may be used for several purposes. These are inoculated with viruses or other pathogens. Haploids showing resistance are selected and proceeded for diploidization and inoculation of the diploid tissue, followed by selection for resistance, and fusion with other haploid protoplasts of the same or other (related) plants, followed by inoculation and selection for resistance. (BSc 2nd Year Biotechnology and Plant Pathology Notes Study Material)
Genetic Engineering Techniques of Importance to Plant Pathology
These are used in relation to disease development and control. Knowledge of the molecular biology of both, the plant and the pathogen is essential. The increased interest in the genetic systems of pathogens like Agrobacterium tumefaciens and cauliflower mosaic virus is due to the reason that they can be used as vehicles of foreign genetic materials into plant genomes and thereby genetically modify the plants. Interest increased, even more, when it was realized that the genetic system of the pathogens themselves and of their antagonists may be modified in ways that may be used to biologically control the plant pathogens.
The techniques used in gene cloning (i.e. isolation and multiplication of an individual gene or gene sequence by inserting it into a bacterium or yeast cell where it can be replicated) are complex and make up the core of the genetic engineering of organisms. The steps in gene cloning are (i) cloning complementary DNA from mRNA, (ii) cloning genes from genome DNA, and (iii) expression of cloned genes.
Vectors used for gene cloning in plants
Vectors are organisms or agents that can transfer genetic material from one organism, called the donor to another organism, called the recipient, in a way that the genetic material will survive and be expressed in the recipient cell. Plasmids of the plant-infecting bacterium Agrobacterium tumefaciens and the plant-infecting double-stranded DNA cauliflower mosaic virus are used as vectors of genetic material into plants. (BSc 2nd Year Biotechnology and Plant Pathology Notes Study Material)
The Ti-plasmid of A. tumefaciens is a circular double-stranded DNA molecule containing up to 200,000 base pairs organized into several genes. When the bacterium comes in contact with a wounded plant cell, the Ti-plasmid is transferred from the bacterium into the cell. A specific region of the plasmid, the Ti-DNA, is transferred from the plasmid to the nucleus of the plant cell and becomes integrated into the plant nuclear genome transcribed. The Ti-plasmid consists of many genes some of been identified.
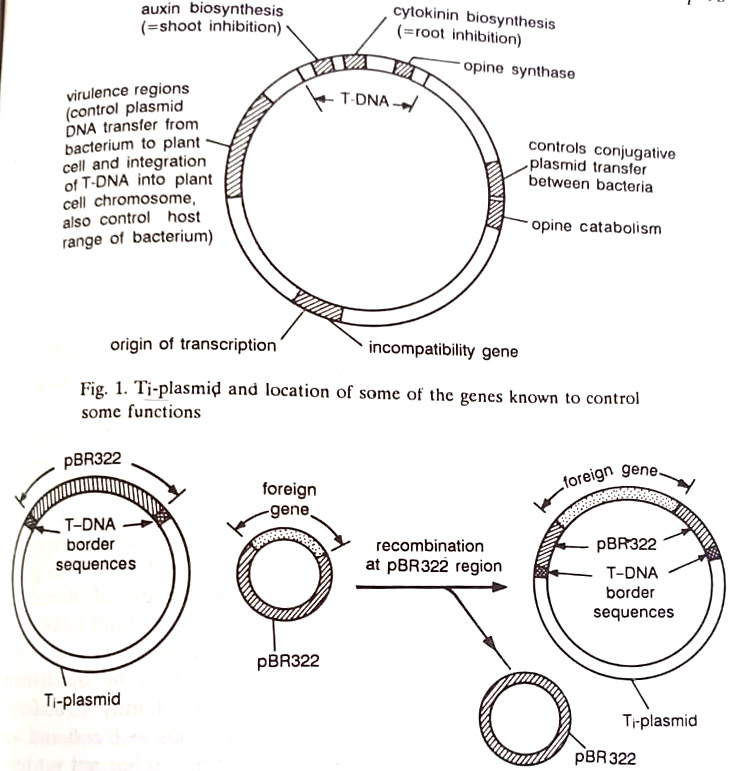
It has now become possible to remove the oncogenic (tumor-inducing) region, containing the genes controlling auxin and cytokinin biosynthesis, from the T-DNA of the Ti-plasmid without removing the border sequences that allow the transfer of T-DNA. Plant cells transformed by such plasmids have lost their independence from plant growth regulators, that is, they are transformed with the new genetic material but are no longer tumor cells and therefore can be regenerated into whole transformed plants.
The problem of the large size of the Ti-plasmid has been solved by using a disarmed, i.e. nontumorous, Ti-plasmid in which the border sequences of T-DNA are preserved but the genes inside the border regions are replaced by a small well understood E.coli plasmid, for example, the one known as PBR 322. The desired foreign gene DNA is also cloned into another PBR 322 plasmid.
By allowing Agrobacterium bacteria to take up both plasmids, homologous recombination between the PBR 322 plasmids takes place within some bacteria and results in the cointegration of the foreign gene in PBR 322 into the T-DNA region of the Ti-plasmid. When bacterial cells containing such plasmids infect plants, they transfer to the plant chromosomes all the DNA between the border sequences of T-DNA, which includes the foreign gene. (BSc 2nd Year Biotechnology and Plant Pathology Notes Study Material)
Such plasmids have already been used successfully to transfer foreign genes into cells that subsequently regenerated into normal, fertile plants and transmitted the introduced genes through meiosis. (BSc 2nd Year Biotechnology and Plant Pathology Notes Study Material)
Transfer of plant, bacterial or animal genes into plants is now possible but little is known of the regulatory controls of expression of such genes. Agrobacterium and the Ti-plasmid infect only dicotyledonous plants, while most major food crops are monocots. Ti-plasmid increased our knowledge of moving genes for resistance from one plant to another, perhaps unrelated plants. The main problem is the identification of the location of resistance genes in plant genomes and the expression of the genes in the introduced plants.
Caulimoviruses have circular double-stranded DNA of about 8,000 base pairs. One best-studied calicivirus to be used as a vector is the cauliflower mosaic virus (CMV). (BSc Biotechnology and Plant Pathology Notes Study Material)
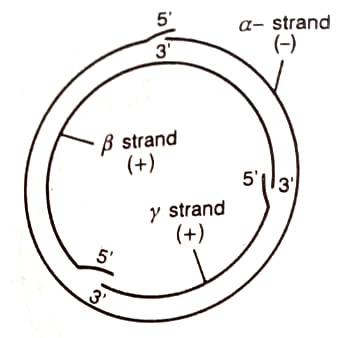
The virus is transmitted by mechanical inoculation of leaves and by aphids to plants in the family Cruciferae. Although the DNA of CaMV has been cloned in plasmids in E.coli and was shown to be infectious after reisolation from the bacteria, it has not yet been possible to use CaMV as a gene vector. (BSc 2nd Year Biotechnology and Plant Pathology Notes Study Material)
BSc 2nd Year Biotechnology and Plant Pathology Notes Study Material
BSc 2nd Year Biotechnology and Plant Pathology Notes Study Material
BSc 2nd Year Sample Model Practice Mock Test Question Answer Papers
BSc 2nd Year Biotechnology and Plant Pathology Notes Study Material