BSc Microbiology of Water Notes Study Material
BSc Microbiology of Water Notes Study Material: BSc is a three-year program in most universities. Some of the universities also offer BSc Honours. After getting enrolled for BSc, there are certain things you require the most to get better grades/marks in BSc. Out of those, there are BSc 2nd Year Study Material, BSc Sample Model Practice Mock Question Answer Papers along with BSc Previous Year Papers. At gurujistudy.com you can easily get all these study materials and notes for free. Here in this post, we are happy to provide you with BSc 2nd Year Microbiology of Water Notes Study Material.

BSc Microbiology of Water Notes Study Material
In the Microbiology of water, we shall briefly examine the main pollutants that make water unfit for drinking and the methods of its purification. Some microbiological tests of water for its safety in consumption will also be described. We shall also examine briefly the sewage treatment since sewage and water are important sources of microbial transmission.
Types of Water
There are two major types of water.
1. Groundwater. It originates from deep wells and subterranean springs. This is virtually free of bacteria due to the filtering action of the soil, deep sand, and rock. However, it may become contaminated when it flows along the channels.
2. Surface water. It is found in streams, lakes, and shallow wells. The air through which the rain passes may contaminate the water. Other sources are the various types of establishments and agricultural farms etc. by the sides the water flows. Possible sources of microbial contamination of a body of water are soil and agricultural runoff, farm animals, rainwater, industrial waste, discharges from sewage treatment plants, and stormwater runoff from urban areas.
In water microbiology, the water is contaminated when it contains a chemical or biological poison or an infectious agent. These conditions also apply to water that is polluted except that the agent or poison is often obvious and the water carries an unpleasant taste or appearance. Portability refers to the drinkability of water. When potable, it is fit for drinking. When unpotable it is unfit due to some contaminant or pollutant. (BSc Microbiology of Water Notes Study Material)
Microbial Components of Water
The types of microorganisms differ in unpolluted, polluted, and marine waters.
We shall briefly examine such differences. Typical organisms in these water environments are shown in Table.

In unpolluted water of mountain lakes or streams, there are usually low organic nutrients. The number of bacteria is very much limited, a few thousand per ml. Actinomycetes are typical. Other organisms shown in the Table are also present. Autotrophic bacteria are common along with free-living protozoans such as Euglena, Paramecium, and various amoebae.
In polluted waters, there are large amounts of organic matter from sewage, feces, and industrial complex. The microbes are usually heterotrophic. The digestion of organic matter by these organisms is incomplete, due to which there accumulate acids, bases, alcohols, and various gases.
The major types of bacteria are coliform bacteria, the Gram-negative non-spore-forming bacilli usually found in the intestine. This group includes E. coli and species of Enterobacter. They ferment lactose to acid and gas. Non-coliform bacteria-Streptococcus, Proteus and Pseudomonas are also present.
Under some conditions, the polluting organisms multiply rapidly and consume most of the available oxygen. For instance, nutrients enter the river from sources like sewage treatment plants or urban/suburban runoff. Thus river suddenly develops a high nutrient content. Under these conditions, algae may bloom rapidly. This leads to the depletion of oxygen in the water.
There is very little oxygen available to protozoa, small animals, fish, and plants. Due to this non-availability of oxygen, a layer of dead organisms, mud, and silt accumulate at the bottom, and anaerobic species of Clostridium, Desulfovibrio, etc. will flourish. They produce gases. One gas, H2S combines with lead or iron to give a precipitate which makes the mud black and the water poisonous.
Due to the complete depletion of oxygen, the suspended bacteria die in their own waste products. There is hardly any life in the water at this stage. The gas bubbles from the anaerobes in the mud break! the surface. Such processes lead to the death of a river.
The marine water is high in salt and only halophilic organisms survive. Since the temperature is very low, most of them are psychrophilic. Diatoms and protozoa as dinoflagellates are important components in the food chain. In the offshore oceanic zone, photosynthetic organisms are present. These are diatoms and dinoflagellates. Most marine microbes are found along the shoreline or littoral zone where nutrients are in abundance. Some unusual types are also present on the ocean floor in the benthic zone and even at the bottom of several miles-deep trenches, the abyssal zone. (BSc Microbiology of Water Notes Study Material)
Water Pollution
Polluted waters can be classified in different ways. In a microbiological context, there is generally recognized physical, chemical, and biological pollution of water. Of these biological pollution is of much concern to us.
1. Physical pollution. Here the water becomes cloudy due to the presence of some particulate matter such as sand or soil. Some plant materials such as algal bloom may also be involved, which turns the consistency of water into a pea soup. The presence of some phosphates or nitrates may encourage the growth of blooms, leading finally to a condition known as eutrophication. This upsets the ecological balance.
2. Chemical pollution. It occurs due to the introduction of inorganic and organic waste to water. For example, water passing out of a mine contains a large amount of copper, iron, or other ore. Another source of pollution is metal pipelines. Corrosion of metals in the pipes may yield rusty or black precipitates. Other chemical pollutants include laundry detergents, radioactive wastes, and nitrates from fertilizers, etc.
An important group of chemicals is polychlorinated biphenyls or PCBs, a group of industrial chemicals used in the production of electric insulators and paper products. They are carcinogenic and necessary to be eliminated from the water. The main sources of chemical pollutants are mining wastes, air pollutants (washed down by rain), power plants, industries, sewage, and stormwater runoff, wash from farmlands (pesticides, fertilizers), hospitals, road salts, and exhausts from automobiles. (BSc Microbiology of Water Notes Study Material)
3. Biological pollution. It develops from the microorganisms that enter waters from such sources as human waste, food operations, meat-packing plants, medical facilities, etc. Under normal conditions, the water body is able to handle biological material, because heterotrophs digest the organic matter into simple phosphates, nitrates, and sulphates which fertilize the plants. Carbohydrates are converted to CO2 and water, which are also useful to plants. The plants then release O2 through photosynthesis. Due to the rapid movement of water, aeration is more or less constant and the wastes are eliminated. (BSc Microbiology of Water Notes Study Material)
However, there arises the problem when water becomes stagnant or is overloaded with waste. Water is now unable to handle the biological material, and it soon becomes polluted. We have already seen how a lake may turn into a swamp due to the overabundance of organic matter. A critical factor operating under such a situation is the biological oxygen demand (BOD). It refers to the oxygen requirement by the metabolizing organisms. As the number of organisms increases, the demand for O2 increases proportionally. Depletion of O2 in water may lead to the death of fish and other aquatic animals, a flat taste of water, and the eventual death of aerobes.
The BOD is therefore an important indication of the levels of biological pollution in the water. In the laboratory, BOD is determined by measuring the amount of dissolved oxygen in test samples of water distributed to BOD bottles. The initial determination of dissolved oxygen in the sample is made. The bottles are then stoppered and incubated at 20°C for five days, after which the amount of dissolved oxygen is again determined. The difference in the oxygen content represents the amount of oxygen used up in the test sample, which is the BOD. Results are expressed as parts per million (ppm). A BOD of several hundred taken is usually high.
Diseases transmitted by water
Water is in fact a vehicle for the transfer of a wide range of diseases of microbial origin. The important bacterial diseases include typhoid fever, cholera, and bacterial dysentry which are generally transmitted when human feces from carriers or patients contaminates the water. Viral diseases transmitted by water include viruses hepatitis and polio. Many protozoa form cysts that survive for long periods in water. The causal agents of amoebiasis, giardiasis, etc. are important concerns in water pollution. (BSc Microbiology of Water Notes Study Material)
Water Treatment
We have seen that water and sewage harbor a variety of organisms responsible for diseases in man. It is thus necessary to remove or rather interrupt the disease cycle in nature through the treatment of water and sewage. Water purification and sanitary sewage disposal are two major methods for interrupting the disease cycle. In water purification organisms are prevented from reaching the body whereas, in sewage disposal, they are removed from body waste products. (BSc Microbiology of Water Notes Study Material)
The various steps in the purification of drinking water in municipal water supplies are shown in Figure. It may be seen that there are three basic steps in the purification of drinking water: sedimentation, filtration, and chlorination.
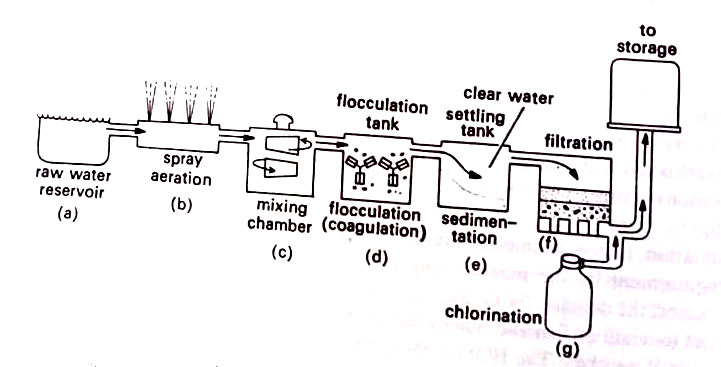
1. Sedimentation. The objective is to remove bulky objects such as leaves, sand, and gravel particles and the materials that have run off from the soil. (BSc Microbiology of Water Notes Study Material)
Sedimentation is done in large reservoirs or in a restricted area of a settling tank. Aluminum sulphate (alum) or iron sulphate may be added to hasten the sedimentation process. This is done in a mixing chamber (C) in Fig. After mixing, the chemicals form jellylike masses of coagulated material called flocs. They fall through the water and cling to organic particles and microorganisms, dragging a major portion to the bottom sediment in a process called flocculation. Coagulated material is allowed to settle in a settling tank.
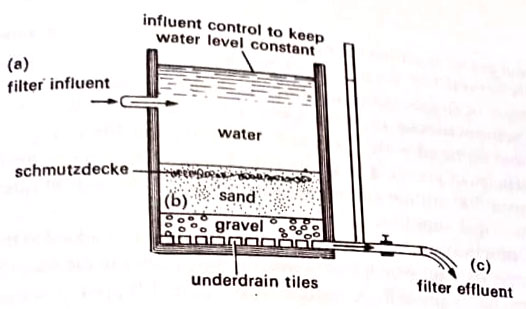
2. Filtration. This process removes the remaining microorganisms from the water. Although different types of filter material are available, mostly a layer of sand and gravel is utilized to trap the microorganisms. A slow sand filter uses several feet-deep layers of fine sand particles. Within the sand there forms a layer of microorganisms that acts as an additional filter. This layer is called a Schmutzdecke or dirty layer.
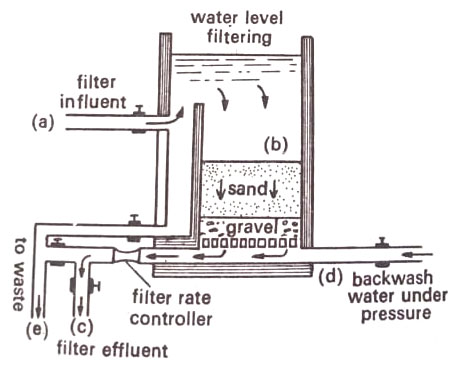
To cleanse the filter, the top layer is removed and replaced with fresh sand. A rapid sand filter contains coarser particles of gravel. The schmutzdecke does not develop; the filtration rate is higher (over 200 million gallons per acre per day). This type of filter is generally used in municipal supplies. (BSc Microbiology of Water Notes Study Material)
3. Chlorination. In this final step, chlorine gas is added to the water. This gas is an active oxidant that reacts with organic matter in the water. Gas is added until any residue is present. A residue of 0.2 ppm-1.0 ppm of water is often the standard used. Under these conditions, most remaining microbes die within 30 minutes. (BSc Microbiology of Water Notes Study Material)
Bacteriological Analysis of Water
There are methods to detect bacterial contamination of water. The chief objective is to identify coliform organisms such as Escherichia coli. Their presence indicates that water contains fecal pollution and is unsafe for consumption. The standard methods used are as follows:
1. Membrane filter technique. It may be used in the field also. A special, collecting bottle is held against the water current and a 100-ml sample is taken. The water is filtered through a membrane filter and the filter pad is then transferred to a plate of the bacteriological medium. Bacteria trapped in the filter will form colonies that may be counted. (BSc Microbiology of Water Notes Study Material)
2. Standard plate count (SPC). A water sample is diluted in the sterile buffer. Measured amounts are pipetted into Petri dishes. Agar medium is added and plates are incubated. Colony counts are made and multiplied by the reciprocal dilution factor to have total bacteria per ml of water. This method is similar to that used for milk. (BSc Microbiology of Water Notes Study Material)
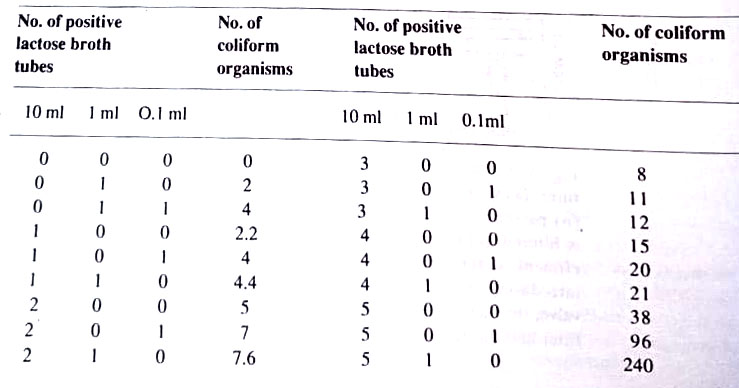
3. Most probable number (MPN). In this procedure, water in 10 ml, 1 ml, and 0.1 ml amounts is inoculated into lactose broth tubes. The tubes are incubated and coliform organisms may be identified by their production of gas from the lactose. By referring to an MPN table, a statistical range of the number of coliforms may be determined by observing how many broth tubes showed gas. This method does not detect the total number of bacteria in the water nor it locates non-coliforms like Salmonella. However, it indicates the presence and quantity of coliforms. (BSc Microbiology of Water Notes Study Material)
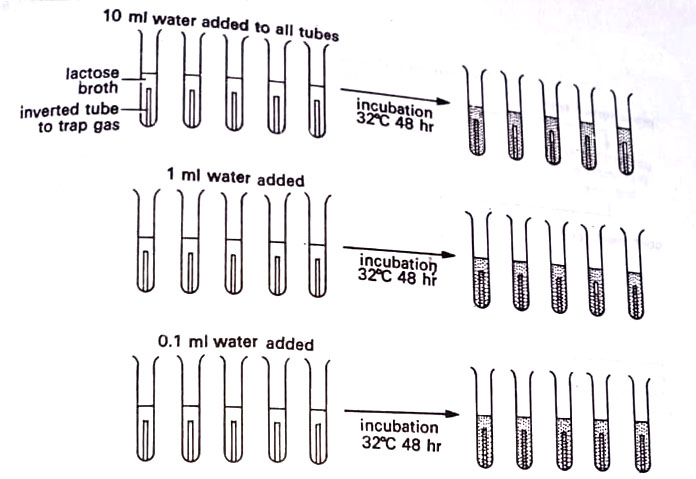
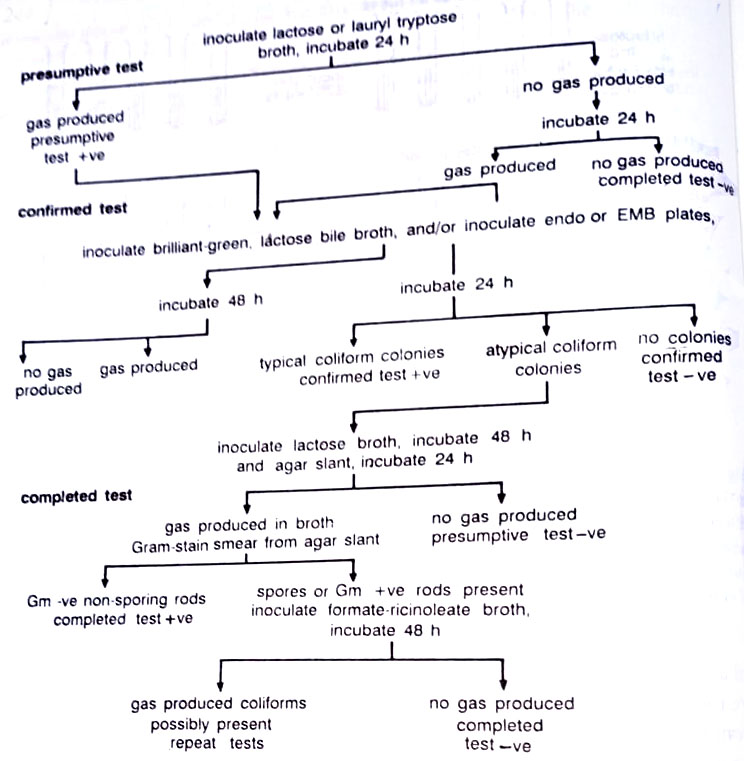
4. Specific tests (Coliform counts) for assessing water safety. The most frequently used indicator organism is the normally nonpathogenic coliform bacterium Escherichia coli. Positive tests for E. coli do not prove the presence of enteropathogens, such as Salmonella and Shigella, but do establish the possibility. E. coli are more numerous and easier to be grown. For fecal contamination of water, using E. coli as an indicator organism, a three-stage test procedure is followed. (BSc Microbiology of Water Notes Study Material)
(a) Presumptive test. In the first stage, lactose broth tubes are inoculated with undiluted or appropriately diluted water samples. The tubes showing gas formation are marked as positive and used to calculate the most probable number (MPN) in the sample. Gas formation in inverted test tubes (Durham tubes), indicates fecal contamination by coliforms.
(b) Confirmed test. Gas formation in lactose broth at 37°C is characteristic not only of Salmonella, Shigella, and E. coli strains but also of the non-fecal coliform Enterobacter aerogenes and some Klebsiella spp. In the second stage, the presence of enteric bacteria is confirmed by streaking samples from the positive lactose broth culture onto a medium, such as eosin methylene blue (EMB) agar. Fecal coliform colonies on this medium acquire a greenish metallic sheen, Enterobacter spp. form reddish, and non-lactose fermenters form colorless colonies respectively. (BSc Microbiology of Water Notes Study Material)
(c) Completed test. The confirmed test can be accomplished by using brilliant green lactose-bile broth (BGLB). If BGLB is used, it is then subcultured onto EMB. Sub-culturing colonies showing a green metallic sheen on EMB into lactose broth incubated at 35°C should produce gas, completing a positive test for fecal coliforms. (BSc Microbiology of Water Notes Study Material)
BSc Microbiology of Water Notes Study Material
BSc 2nd Year Sample Model Practice Mock Test Question Answer Papers