BSc 2nd Year Control of Microorganisms Notes Study Material
BSc 2nd Year Control of Microorganisms Notes Study Material: We provide to all the students BSc 1st, 2nd, and 3rd Year notes Study material, question answers, sample papers, mock test papers, and pdf. At gurujistudy.com you can easily get all these study materials and notes for free. Here in this post, we are happy to provide you with BSc 2nd Year Control of Microorganisms Notes Study Material.

BSc 2nd Year Control of Microorganisms Notes Study Material
The control of microorganisms is necessary for good health. In this post, we shall briefly consider some of the established methods of their control. There are three principal kinds of agents used in control : (i) physical agents, which are used exclusively on objects outside the body, (ii) chemical agents, which are used on inanimate objects as well as on the body surface and (iii) chemotherapeutic agents, which are most often used inside the living body.
Physical Control
The chief agent used in this type of control is heating, applied in different forms. In addition, there are some other methods also, in which different types of time physical agents are used.
1. Heat. The aim is often sterilization. This term implies the removal of all life forms and this is an absolute term. Following are the common ways in which heat is used in control.
(a) Direct flame. This method is used in the process of incineration, employed in the laboratory to sterilize the bacteriological loop and needle before removing a sample from a culture tube and after preparing a smear. The tip of the tube is also flamed to destroy microbes that may contact it.
(b) Hot-air oven. This instrument utilizes dry heat for sterilization. The material is put at 160°C for a period of two hours to kill the bacterial spores and other microbial structures. This method is often used to sterilize dry powders, oily substances, and glassware.
(c) Boiling water. Here the objects are immersed in boiling water. The moist heat sterilizes the objects. This heat penetrates better and more rapidly than dry heat. Since the temperature is 100°C, bacterial spores may require two hours of exposure for destruction. Moist heat kills microorganisms by coagulating and denaturing their proteins. The object must be completely immersed in boiling water.
(d) Autoclave. The principle used here is to increase the pressure of steam (gas) in a closed system that increases its temperature. The water molecules become more aggregated which increases their penetration considerably. This principle is used to reduce sterilizing time in the laboratory instrument called the autoclave. It contains a sterilizing chamber into which articles are placed and a steam jacket where steam is maintained. A special valve increases the pressure to 15 lb/sq. an inch above normal atmospheric pressure.
The temperature rises to 121.5°C and superheated water molecules rapidly conduct heat into the microorganisms within about 15 ms. Culture media, glassware, metalware, etc.
(e) Fractional sterilization. It is also called vandalization (after its developer, John Tyndall), and intermittent sterilization as it is a stop-and-start operation.
Sterilization is achieved by a series of events. During the first day, objects are exposed to free-flowing steam at 100°C for 30 min. which kills all organisms except bacterial spores. When left overnight the spores germinate into vegetative cells. These are killed during the second day’s exposure to steam for 30 min.
Again, the material is cooled, and the few remaining spores germinate, which are killed on the third-day exposure. This method is particularly important in modern microbiology, for sterilizing those objects which could not be sterilized by autoclave. The instrument used is the Arnold sterilizer.
(f) Pasteurization. This is not supposed to provide sterilization. Here the bacterial population of a liquid such as milk is merely reduced, and the organisms which may cause human diseases are destroyed. Spores are not affected. One method for milk pasteurization is the holding method. It involves heating at 62.9o C for 30 min. It kills both tubercle bacterium, Mycobacterium tuberculosis, and the Q fever agent. Other methods are flash pasteurization at 71.0°C for 15 seconds, and ultrapasteurization at 82°C for 3 seconds.
(g) Hot oil. Some physicians and dentists use hot oil at 160°C for one hour for sterilization of the instruments.
2. Other methods. Physical methods other than heat, are as follows:
(a) Filtration. It is a mechanical device for removing microorganisms from a solution. The organisms are trapped in the pores of the filter, and the filtrate is decontaminated or possibly sterilized. There are several types of filters used in the microbiology laboratory. Inorganic filters are typified by the Seitz filter, which consists of a pad of asbestos mounted in a filter flask. Porcelain and ground glass may also be used. There are organic filters also. The organic molecules of the filter attract the organic components of the microorganisms.
An example, the Berkefeld filter, utilizes the substance-diatomaceous earth, composed of the skeletal remains of marine alga called diatoms. The third type of filter is the membrane filter which received general acceptance. It consists of a pad of organic compounds such as cellulose acetate or polycarbonate mounted in a holding device. Membrane filters are available in various pore sizes according to the microorganisms to be trapped. This method can be used for the quantitative estimation of microbes in a given sample. The filter pad is put on a nutrient culture medium where cells grow. (BSc 2nd Year Control of Microorganisms Notes Study Material)
(b) Ultraviolet light. UV light has a wavelength between 100 and 400 nm, and the energy at about 265 nm is most destructive to bacteria. Exposure to UV damages the DNA. UV radiation are used to reduce air contamination.
(c) Other radiations. The spectrum of energies with wavelengths less than that of UV includes—X-rays and gamma rays which can also be used to destroy bacteria. These rays are called ionizing radiations as they eject electrons out of organic molecules, thus creating ions.
(d) Ultrasonic vibrations. These are high-frequency sound waves. If propagated in fluids, sound waves cause microscopic bubbles to form, and the water appears to boil (also called cold boiling). The bubbles rapidly collapse, giving tiny cavities and sending out shock waves. Microorganisms in the fluid are rapidly disintegrated by external pressures. A device called cavitron is used by dentists to clean teeth. There are ultrasonic machines to clean dental plates coins, jewelry, etc.
(e) Preservation methods. A number of physical methods are used for controlling microorganisms in foods. Thus food spoilage is checked. These are as follows:
i. Drying. It is used for the preservation of meat, cereal, fish, and other foods.
ii. Salting. It is based on the principle of osmotic concentration. In presence of salt, water comes out of cells due to exosmosis, causing ultimate death. This method is used in the preservation of syrups, jams, jellies, etc. from bacterial contamination.
iii. Low temperature. In the refrigerator and freezer, low temperatures retard spoilage by reducing the metabolic rates of microorganisms.
Chemical Control
Chemical methods are expected to remove the pathogenic organisms from an object (or body). These methods rarely achieve sterilization as in physical methods. The process of removal is called disinfection. If the object is non-living, the chemical is known as a disinfectant. However, if the object is living, as a tissue of the human body, then the chemical is an antiseptic. Antiseptics and disinfectants may be either bactericidal or bacteriostatic. The former agent kills the microbes whereas the latter temporarily prevents their further multiplication.
The effectiveness of a chemical agent is based on the phenol coefficient (PC). This number indicates the ability of a particular antiseptic or disinfectant as compared to phenol under identical conditions. A PC higher than one indicates that the chemical is more effective than phenol.
Following are some of the commonly used chemicals to control microorganisms.
1. Halogens. Two halogens, chlorine, and iodine are most commonly used. Halogens are highly reactive elements. Chlorine is available either as gas or as organic or inorganic compounds. It is used in municipal water supplies, keeping the bacterial population at low levels.
The residue used is about 0.2-1 ppm of free chlorine. One ppm is equivalent to 0.0001 percent. Chlorine is also useful as sodium hypochlorite (NaOCl) or as calcium hypochlorite [Ca (oCl2.)]. Other forms of chlorine are chloramines. Iodine is used generally in the form of a tincture of iodine, as an antiseptic for wounds. It has 2% iodine plus sodium iodide in ethanol. Iodophors are complexes of iodine and detergents that release the iodine over a long period of time. (BSc 2nd Year Control of Microorganisms Notes Study Material)
2. Phenolic compounds. Phenol is the standard disinfectant, which coagulates the proteins, particularly cell membrane enzymes. Phenol is especially useful against Gram-positive bacteria. An alternative to phenol, cresol has become more popular in modern medicine as it is cheaper than phenol. Cresols are used as wood preservatives. Bisphenols—a combination of two phenol molecules are also prominent in modern disinfection and antisepsis. For example, orthophenylphenol is used in Lysol, osyl, staphene, etc.
Another bisphenol, hexachlorophene was used extensively in the 1950s and 1960s in toothpaste, underarm deodorants, and bath soaps. A phenol derivative, hexylresorcinol is used in mouthwash, topical antiseptic, and in throat lozenges.
3. Heavy metals. The activity of heavy metals on microorganisms is termed oligodynamic action. Metals such as silver, mercury, and copper are used, Mercury is an older antiseptic, used as mercuric chloride (HgCl2). In products like mercurochrome, Merthiolate, and metaphen; mercury is combined with organic carrier compounds, which reduces its toxicity to the skin.
Copper is particularly active against algae. It is used as copper sulphate in swimming pools and municipal water supplies, Silver, as silver nitrate is used as an antiseptic and disinfectant. One drop of 1% AgNO3 solution is placed in eyes of newborns to protect against infection by the gonococcus, Neisseria gonorrhoeae. Silver nitrate can also be combined with an antimicrobial drug for use in the treatment of burns.
4. Alcohol. It is an effective antiseptic, applied to the skin. The most common is ethyl alcohol, though propyl, butyl, and pentyl alcohols have the greater germicidal ability. But they are more expensive and do not easily mix with water. Methanol is toxic to tissues. Ethanol acts particularly on vegetative bacterial cells. It is a strong dehydrating agent. Ethyl alcohol (70%) is mostly used.
5. Alkylating agents. Formaldehyde is the most common. This compound is a gas at high temperatures but solid paraformaldehyde at room temperature. Formalin is prepared by suspending 40g of this solid in water. This is used in anatomical specimens. It is also used in the inactivation of viruses in vaccine preparations and in the production of toxoids from toxins. Ethylene oxide (Eto) is used in the sterilization of plastic materials used in laboratories. It is used in combination with freon in a ratio of 12:88, available as cryoxide or steroxide.
Carboxide contains Eto and carbon dioxide. The gas is released into a tightly sealed chamber. Other alkalyting agents are beta-propiolactone and glutaraldehyde. (BSc 2nd Year Control of Microorganisms Notes Study Material)
6. Hydrogen peroxide. It is used as a rinse for wounds, scrapes, or abrasions. New forms of stable H2O2, like Super D hydrogen peroxide, have also appeared.
7. Soaps and detergents. Since the pH of soaps is about 8.0, it destructs microorganisms to some extent due to alkalinity. Soap is used for machine washing the skin surface. Soaps are wetting agents, emulsifying and solubilizing particles that cling to a surface. Detergents are synthetic chemicals developed for their ability to be strong wetting agents and surface tension reducers.
8. Dyes. A group of dyes-triphenyl methane dyes is useful as antiseptics for Bacillus spp. and Staphylococcus spp., and in higher concentration for typhoid bacilli. This group includes malachite green and crystal violet (traditionally used as gentian violet for trench mouth and Candida infections). The second group of dyes, the acridine dyes are good antiseptics for gonococcal and staphylococcal infections. Generally, dyes are more valuable for Gram-positive than for Gram-negative forms. They act by combining directly with DNA and halt RNA synthesis. (BSc 2nd Year Control of Microorganisms Notes Study Material)
9. Acids. Some acids are good disinfectants and antiseptics. The common ones are benzoic, salicylic, and undecylnic acids for tinea infections of the skin. Organic acid as lactic acid and acetic acid are good food preservatives.
Chemotherapeutic Agents
It could become possible only in the 1940s that successful treatment of fatal diseases was achieved. Early efforts of microbiologists toward the control of diseases were mainly centered on enhancing the role of the immune system. Vaccines for rabies, diphtheria, and tetanus were developed. Among the pioneers in this field were Emil von Behring, Elie Metchnikoff, and Paul Ehrlich, all Nobel Prize winners in Physiology or medicine. Ehrlich is created with the development of the first chemotherapeutic agent, who conceived the antibody molecules as “magic bullets”.
In the early 1900s, his attention turned to magic bullets of a purely chemical nature, the chemotherapeutic agents. Ehrlich and his associates had synthesized hundreds of arsenic-phenol derivatives. These were tested by one of his associates, Sahachiro Hata, against the syphilis organism. Later, however, their attention focused on a single chemical, compound 606. This chemical, after trials in animals, was made available to physicians under the trade name Salvarsan which soon became the first useful chemotherapeutic drug.
The technical or generic name was arsphenamine. Ehrlich’s death in 1915 and the emerging World War eroded the enthusiasm for chemotherapy. Further progress in this area was made only after about 20 years. German chemists developed some industrial dyes, that exhibited antimicrobial qualities also. A red dye, prontosil was synthesized in 1932, that showed activity against some Gram-positive bacteria such as streptococci and staphylococci. (BSc 2nd Year Control of Microorganisms Notes Study Material)
In 1935, a French group headed by Jacques and Thevese Trefouel announced that sulfanilamide was the active component of prontosil. Gerhard Domagk was awarded the Nobel Prize in 1939 for the successful treatment of war-related infections with sulfanilamide.
Following are the common groups of chemotherapeutic agents used in the control of infectious microorganisms:
[I] Sulfanilamide and other sulfonamides
Sulfanilamide was the first of a group of chemotherapeutic agents known as sulfonamides. They interfere with the metabolism of bacteria through the mechanism known as competitive inhibition. Modern sulfonamides are typified by sulfamethoxazole, prescribed for urinary tract infections due to Gram-negative rods. The drug is combined with trimethoprim, available commercially under the trade name Bactrim. There are two kinds of names for each chemical, generic name and trade name.
Other common sulfonamides are, sulfacetamide, sulfabenzamide and sulfathiazole, commercially available as Triple Sulfa. This is used for vaginal infections due to Haemophilus sp. Another sulfonamide, sulfisoxazole is marketed as a cream for vaginal infections.
[II] Other chemotherapeutic agents
There are many other agents, which became common these days. They are not related to sulfonamides. Some common ones include isonicotinic acid hydrazide, effective for tuberculosis: trimethoprim used in urinary tract infections; nalidixic acid for some Gram-negative bacteria of urinary tract infections and nitrofurantoin, also used for such infections.
Metronidazole (Flagyl) is effective against Trichomonas vaginalis infections and amoebiasis. However, there is evidence that it causes tumors in mice Primaquine destroys the malarial parasite. Dapsone is used in the treatment of leprosy.
[III] Antibiotics
Alexander Fleming was a student of Almroth Wright, the British investigator who described opsonins. Fleming described the nonspecific enzyme. lysozyme. In 1928, he observed that a Petri dish culture of staphylococci had become contaminated with a green mold and that the bacteria were disappearing as the mold grew over the plate. The mold isolated was identified as Penicillium and found that the broth contained an active principle with antibacterial characteristics. Although he failed to isolate the substance, he called it penicillin.
He recognized the mold’s potential for the treatment of human diseases, trying filtrates on infected wounds. Rene Dubos in 1939 indicated that soil bacteria could produce antibacterial chemicals like Fleming’s penicillin. A group at Oxford University, led by the British pathologist, Howard Florey and the German biochemist, Ernst Boris Chain, reisolated penicillin and carried out careful trials with highly purified samples. In 1940, their successful attempts were published.
American pharmaceutical companies developed technology for the large-scale production of penicillin. Fleming, Florey, and Chain received the Nobel prize in 1945 for the discovery and development of penicillin. Since this was a naturally occurring product, the term antibiotic was introduced in medicine. (BSc 2nd Year Control of Microorganisms Notes Study Material)
Following are some of the most commonly used antibiotics:
1. Penicillin. A large group of penicillin derivatives is available. Penicillin G or benzylpenicillin is the most popular of the penicillins. Other types are penicillin F or penicillin V. All have the basic same structure with a beta-lactam nucleus and several attached groups as shown in Figure.
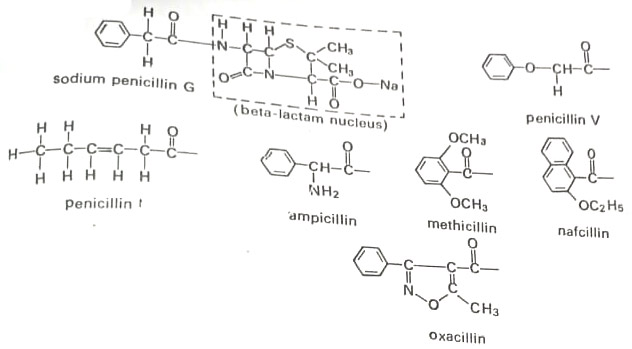
Penicillins are active against a variety of Gram-positive bacteria, including staphylococci and streptococci. Penicillin functions during the synthesis of the bacterial cell wall. The molecule blocks the cross-linking of hexoses in the peptidoglycan layer during wall formation, causing the bursting of the cell. There are, however, two major drawbacks to the use of penicillin.
One is the anaphylactic reaction in those who are allergic to penicillin. It causes swelling in the eyes or wrists, itchy skin, etc. Second, the evolution of some penicillin-resistant bacteria that produce an enzyme, penicillinase. This converts penicillin into harmless penicilloic acid. Modern penicillins are mostly produced from Penicillium notatum and P. chrysogenum. (Control of Microorganisms Notes Study Material)
2. Semisynthetic penicillins. In the late 1950s, the beta-lactam nucleus of the penicillin molecule was identified and synthesized. Various groups than could be attached to this nucleus, creating a number of new penicillins. At present thousands of penicillins are prepared by this semi-synthetic process. Ampicillin is less effective than Penicillin G against Gram-positive cocci but valuable against some Gram-negative rods. It can be taken orally and absorbed from the intestine. Amoxicillin is similar to penicillin.
Both are useful to treat urinary tract infections. Another semisynthetic penicillin, carbenicillin is used for Pseudomonas and Proteus infections of the urinary tract. Others are methicillin, nafcillin, and oxacillin, which are resistant to penicillinase.
3. Cephalosporins. They were developed in the 1960s. Cephalosporin C was isolated from the blue mold. Cephalosporium. A number of related semisynthetic drugs developed from it are cephalexin, cephalothin, cefazolin, and cephaloridine. Cephalosporins are alternatives to penicillin and are effective for staphylococcal boils or wounds, streptococci, and bacterial pneumonia, and urinary tract infections by Gram-negative bacteria. (BSc 2nd Year Control of Microorganisms Notes Study Material)
4. Streptomycin (an aminoglycoside). This was discovered by Selman A. Waksman. Several moldlike soil bacteria-actinomycetes produced antimicrobial chemicals. Of these, Streptomyces griseus was the most effective producer. The substance produced by it was named Streptomycin by Waksman, Elizabeth Bugie, and Albert Shatz. This drug was made available in 1947 and Waksman received Nobel Prize in 1952 in Physiology or medicine. Streptomycin in combination with isoniazid is important for the treatment of tuberculosis. Gram-negative infections such as plague and brucellosis are also treated. (BSc 2nd Year Control of Microorganisms Notes Study Material)
5. Other aminoglycoside antibiotics. Gentamicin is the first drug to be given for infections by Gram-negative bacteria. It is combined with carbenicillin for Pseudomonas infections, with ampicillin for streptococcal infections of the intestine, and with cephalosporin for staphylococcal disorders. Neomycin is now used as a topical antibiotic for eye bacterial conjunctivitis or other Gram-negative infections.
Commercially, it is available as Neosporin when combined with polymyxin, and as cortisporin when combined with cortisone, bacitracin, and polymyxin. The mixtures are useful for a variety of mild skin infections due to Gram-negative or Gram-positive bacteria. All aminoglycosides are derived from the species of Streptomyces. (BSc 2nd Year Control of Microorganisms Notes Study Material)
6. Chloramphenicol. This is the first broad-spectrum antibiotic discovered. It was isolated in 1947 by Ehrlich, Burkholder, and Gotlieb. It inhibits a wide variety of Gram-positive and Gram-negative bacteria, as well as several rickettsiae and fungi. Chloramphenicol was originally isolated from the metabolites of Streptomyces venezuelae. However, there are two main disadvantages to its use.
In the bone marrow, it prevents hemoglobin incorporation into the red blood cells—aplastic anemia. Moreover, due to its accumulation in the blood of a newborn child, it causes a toxic reaction and sudden breakdown of the cardiovascular system-gray syndrome.
7. Tetracyclines. They are also broad-spectrum antibiotics with a range of activity similar to chloramphenicol. They include naturally occurring chlortetracycline and oxytetracycline, isolated from species of Streptomyces. They may be taken orally.
Though they have side effects problems, they remain the drugs of choice for most rickettsial and chlamydial diseases. They are used in Gram-negative infections such as brucellosis, plague, and cholera, for primary atypical pneumonia, as substitutes for penicillin in syphilis, anthrax, gonorrhea, and pneumonia; and as therapy of some protozoan infections such as amoebiasis.
8. Other antibiotics. Erythromycin, obtained from Streptomyces is useful for primary atypical pneumonia, staphylococcal and streptococcal infections, and syphilis. Vancomycin, also a product of Streptomyces is given intravenously against Gram-positive infections. Other antibiotics are rifampin for leprosy and tuberculosis, clindamycin, and lincomycin, active against streptococci staphylococci and other Gram-positive organisms. (BSc 2nd Year Control of Microorganisms Notes Study Material)
Bacitracin and polymyxin, obtained from Bacillus species are used topically. The former is used as an ointment for staphylococci, and the latter for Gram-negative bacilli. Spectinomycin, a product of Streptomyces became popular in the late 1970s as a substitute for penicillin in case of gonorrhea caused by PPNG. It is given intramuscularly. (BSc 2nd Year Control of Microorganisms Notes Study Material)
9. Antifungal antibiotics. Nystatin, a product of Streptomyces is used as cream or ointment or in suppository form, for infection of the oral cavity, vagina, or intestine due to Candida albicans. Griseofulvin is used for fungal infections of the skin, hair, and nails. It is effective against ringworm and eczema. This is a product of Penicillium.
For serious systemic fungal infections, amphotericin B is used. This is effective for organisms of histoplasmosis, blastomycosis, cryptococcosis, etc. (BSc 2nd Year Control of Microorganisms Notes Study Material)
Mode of Action of Antibacterial Agents
Knowledge of the mode of action of antibiotics should help design new and better chemotherapeutic agents.
It may be seen that antibacterials act at different sites of the pathogenic bacterium to which they are applied. They may act accordingly as follows:
1. Cell-wall inhibitors. These include two widely used classes of antibiotics, penicillins, and cephalosporins. Both contain a ß- lactam ring. They act on various Gram-positive and Gram-negative rods and cocci, responsible for various diseases. They inhibit the formation of peptide cross-linkages within the peptidoglycan backbone of the cell wall. Other cell wall inhibitors are vancomycin, bacitracin, and cycloserine.
2. Protein synthesis inhibitors. Such antibiotics are streptomycin, gentamicin, neomycin, kanamycin, tobramycin, and amikacin. These are called aminoglycoside antibiotics and are used for Gram-negative bacteria. They bind to the 30s ribosomal subunit of the 70s prokaryotic ribosome. In addition to these, a number of other antibiotics inhibit protein synthesis. These are tetracyclines, chloramphenicol, erythromycin, lincomycin, clindamycin, and spectinomycin. Chloramphenicol, unlike others, acts primarily by binding to the 50s ribosomal subunit, preventing the binding of tRNA molecules to both the amino-acyl and peptidyl binding sites of the ribosome. Erythromycin also binds to 50s ribosomal subunits.
3. Membrane transport inhibitors. Polymyxins, such as polymyxin B changes the structure of the cell membrane and causes leakage of cell contents of Gram-negative bacteria. Pseudomonas spp and other Gram-negative bacteria, resistant to penicillins and aminoglycoside antibiotics are controlled by polymyxins B and E. (BSc 2nd Year Control of Microorganisms Notes Study Material)
4. DNA inhibitors. They block DNA replication. In particular, quinolones interfere with DNA gyrase, preventing the establishment of a replication fork. The quinolones include nalidixic acid, ciprofloxacin, norfloxacin, gatifloxacin, and enoxacin, effective against a range of Gram-positive and Gram-negative bacteria. (BSc 2nd Year Control of Microorganisms Notes Study Material)
5. Other sites of inhibition. Sulphonamides, sulphones, and para-aminosalicylic acid are structural analogues of the vitamin para-aminobenzoic acid. These analogues are effective competitors with the natural substrate for the enzymes involved in the synthesis of folic acid, as such, and thus inhibit the formation of this required co-enzyme (folic acid) causing a bacteriostatic effect.
Trimethoprim is an inhibitor of dihydropholate reductase. DihyTrofolic acid is a co-enzyme required for 1-carbon transfers, as those required in the synthesis of thymidine and purines. This antibiotic is a broad-spectrum one used to treat bacterial infections of urinary and intestinal tracts. (BSc 2nd Year Control of Microorganisms Notes Study Material)
Antibiotic Sensitivity Assays
These assays are used to study the inhibition of a test organism by one or more antibiotics or other chemotherapeutic agents. Two general methods are commonly used:
[I] Tube dilution method
This is often used to determine the smallest amount of antibiotic necessary to inhibit a test organism. This amount is known as the minimum inhibitory concentration (MIC). A set of tubes with different concentrations of a particular antibiotic are prepared.
The tubes are inoculated with the test organism, incubated, and examined for the growth of bacteria. Growth is seen to diminish as the concentration of antibiotics increases, and eventually, an antibiotic concentration may be observed at which growth fails to occur. This is the MIC. (BSc 2nd Year Control of Microorganisms Notes Study Material)
[II] Agar diffusion method
The principle used here is that antibiotics will diffuse from a paper disc or small cylinder into an agar medium that contains test organisms. Inhibition is observed as a failure of the organism to grow in the region of the antibiotic.
A common application of this method is the Kirby-Bauer test, developed in the 1960s. The procedure is used to determine the sensitivity of an organism isolated from a patient to a series of antibiotics. The results serve as a guide for physicians to prescribe a drug.
An agar medium such as Mueller-Hinton medium is inoculated with the organism and poured onto the plate. Paper discs containing known concentrations of antibiotics are applied to the surface, and the plate is incubated. The appearance of a zone of inhibition surrounding the disc is indicative of sensitivity. (BSc 2nd Year Control of Microorganisms Notes Study Material)
By comparing the diameter of the zones to a standard table, one may determine if the test organism is susceptible, or resistant to the antibiotic. If the organism is susceptible, it is likely to be killed in the bloodstream of the patient if that concentration of the drug is reached. Resistance indicates that the antibiotic will u be effective at that concentration in the bloodstream.
BSc 2nd Year Control of Microorganisms Notes Study Material
BSc 2nd Year Sample Model Practice Mock Test Question Answer Papers