BSc Microbiology Microbial Metabolism Notes Study Material
BSc Microbiology Microbial Metabolism Notes Study Material: BSc is a three-year program in most of universities. Some of the universities also offer BSc Honours. After getting enrolled for BSc, there are certain things you require the most to get better grades/marks in BSc. Out of those, there are BSc Study Material, BSc Sample Model Practice Mock Question Answer Papers along with BSc Previous Year Papers. At gurujistudy.com you can easily get all these study materials and notes for free. Here in this post, we are happy to provide you with BSc 2nd Year Microbiology Microbial Metabolism Notes Study Material.
BSc Microbiology Microbial Metabolism Notes Study Material
Term metabolism refers to the sum total of all biochemical transformations that occur in the cells. It is in fact the chemistry of life. This chemistry is generally divided into two sections: anabolism and catabolism.
Anabolism or biosynthesis. It includes all such transformations that are involved in the synthesis of organic macromolecules, making the major portion of cellular mass, from the simpler compounds present in the extracellular environment. Anabolism is normally an energy-utilizing process. Energy is used in the form of ATP. (BSc Microbiology Microbial Metabolism Notes Study Material)
Catabolism. The mechanisms of generating ATP are diverse among organisms. The ATP required for anabolism may be produced by the process of photosynthesis. However, chemical energy in the form of inorganic or organic compounds (that are degraded) can also be used to drive biosynthesis. Such processes, involving the direct use of chemical energy (obtained from the breakdown of chemical compounds inorganic or organic), are termed catabolism or degradative metabolism. Thus catabolism is characterized by the release of energy.
The three important groups of organic compounds involved in metabolism are carbohydrates, lipids, and proteins. In the fourth group, the nucleic acids remain unchanged for long periods of time in the cell.
Enzymes, Energy
In order to drive the chemical processes and reactions, a series of protein molecules, the enzymes must be available. They bring about chemical changes while remaining essentially unchanged. They are said to catalyze chemical changes and once the change has occurred, the enzyme is released to participate in another chemical reaction, as shown in Figure. They are highly specific in their activity. The substance to be changed is called the substrate and the products formed as end products. The mechanism of enzyme action is thought to be a lock and key mechanism, where the enzyme fits into the substrate and then separates it.
In all chemical reactions, the energy is in the chemical form of ATP adenosine triphosphate. Enzymes are supplemented by the chemical energy in ATP molecules. ATP releases its energy by splitting into a PO ion and ADP.
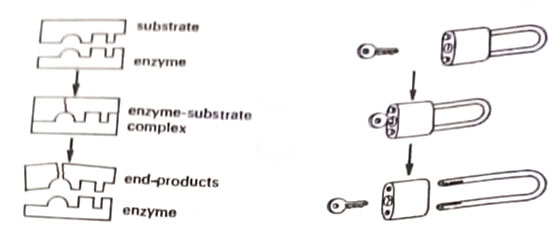
Each time this occurs, about 10000 calories of energy per molecule of ATP are released for cell use.
Generation of ATP
ATP is generated by using the energy released in the catabolism of organic or inorganic compounds to chemically recombine ADP and PO4. ATP is then used to continue driving the reactions of catabolism as well as for anabolism and other activities.
In cellular metabolism, ATP is generated by two fundamentally different biochemical mechanisms: (i) substrate-level phosphorylation and (ii) electron transport or oxidative phosphorylation.
Substrate level phosphorylation. The ATP is formed from ADP by the transfer of a high-energy phosphate group from an intermediate of the catabolic pathway. For example, during the conversion of 2 – phosphoglyceric acid to pyruvic acid in glycolysis.
Electron transport. These ATP-generating mechanisms (oxidative phosphorylation) occur in the membranes. There are involved transfers of electrons between certain carrier molecules with fixed orientation in the membrane.
Metabolism Rates
One of the most important characteristics of microorganisms is their high rate of growth. Many microbes may have a generation time of fewer than 30 minutes and this depends upon the ability to synthesize their own weight of cell material in this period. In other words, they have a correspondingly high rate of metabolism. There exists an inverse relationship between the rates of metabolism or growth and the size of an organism. (BSc Microbiology Microbial Metabolism Notes Study Material)
For example, if we take the rate of oxygen uptake per unit mass as a measure of a man is taken as unity, the following levels are obtained:
Elephant – 0.2
Man – 1.0
Mouse – 10
Microbe:
Eukaryote – 100
Prokaryote – 1000
How is this high rate of microbial metabolism maintained? One of the important factors is the rate of uptake of nutrients and removal of the waste product < the processes which both occur at the cell surface. The smaller the organism, the greater will be its ratio of surface area to volume, or to weight, and hence the easier it will be for it to have a high rate of metabolism and growth.
Higher organisms have developed special mechanisms to increase their useful surface area such as intestines, lungs, kidneys, bloodstream, etc.
Stages of Metabolism
In order to synthesize the main groups of cell constituents i.e. proteins, nucleic acids, polysaccharides, lipids, peptidoglycans, and others, the metabolism of the cell of a microorganism must be directed to the synthesis of the following compounds:
20 L – amino acids, 2-4 peptidoglycan amino acids (in prokaryotes only), 5 purines and pyrimidines, c. 10 monosaccharides, c.10 lipids, c.20 coenzymes and a variety of other essential components.
The minimum number of organic compounds that must be either synthesized by the cell or provided in the environment as monomers, coenzymes and other cell constituents is between 100 and 200. In most microorganisms, the majority of these can be synthesized by the organism itself. The stages of this metabolism are as follows:
(1) The provision of basic carbon intermediates, or building blocks from external carbon sources. It is at this level that all living organisms are divided into two major groups: (i) heterotrophs and (ii) autotrophs. In heterotrophs, the sources of carbon and energy (for biosynthesis) are generally the same organic substances which are broken down partly to supply building blocks and partly to provide energy.
In these organisms, therefore, there is a dual flow of carbon – part emerging as catabolic end products and no drawn-off in the form of intermediates of biosynthetic pathways. In autotrophs, the carbon source is CO2. Energy is consequently not available from the catabolic metabolism of organic substances and must therefore be provided by another mechanism: for example, photosynthesis by phototrophs, or the oxidation of inorganic compounds by chemotrophs. In these organisms (cf. heterotrophs), however, carbon flow leads exclusively to biosynthesis, producing certain biosynthetic intermediates by the sequence of reactions that generate ATP for heterotrophs.
These organisms cannot oxidize exogenous organic substrates, and consequently, the tricarboxylic acid cycle (TCA cycle) does not play a role in generating ATP. Nevertheless, the organisms synthesize all the enzymes of the cycle except ketoglutarate de-hydrogenase, The absence of this enzyme destroys the cyclic nature of the system but it does not affect its biosynthetic function.
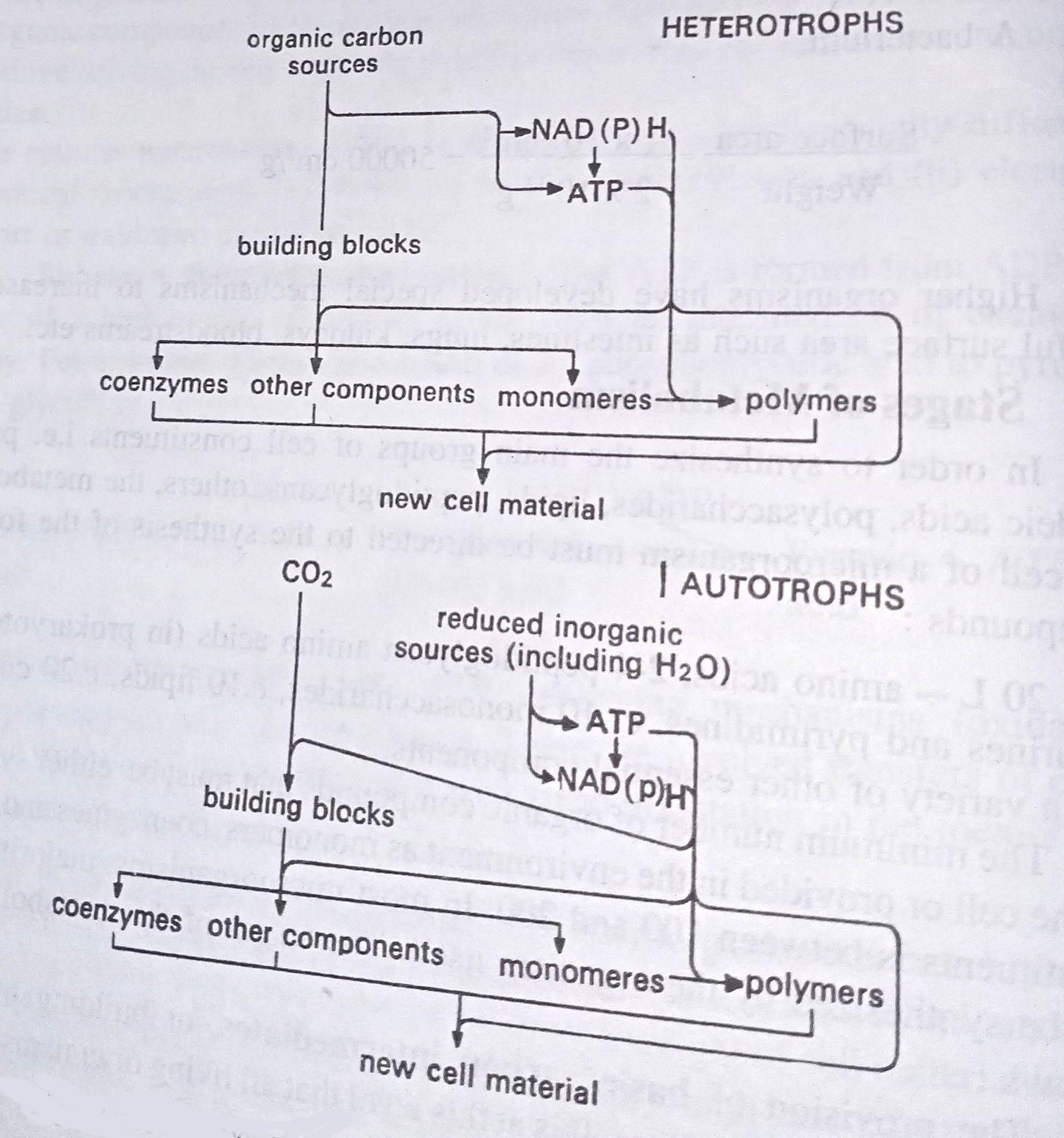
The distinction is clear enough for plants (autotrophs) and for animals (heterotrophs), for microorganisms the borderlines between the various nutritional groups can be blurred with many showing environment-dependent variation in the determinative characteristics
(2) The provision of energy by the formation of ATP, generally from ADP or occasionally from AMP.
(3) The provision of reducing equivalents in the form of reduced pyridine nucleotides; either NADH or NADPH. and referred to here as NADPH. In heterotrophs NAD(P)H is generated along with ATP during the catabolism of the organic carbon sources. In autotrophs the provision of NAD(P)H parallels that of ATP, arising from the photolytic splitting of water in plant and algal photosynthesis, and from the oxidation of reduced inorganic compounds in the bacterial photo- and chemoautotrophs.
(4) The conversion of these building blocks into monomers, coenzymes, and other cell components.
(5) The polymerization of the monomers.
(6) The formation of the quaternary structure of the polymers, together with other essential components, and their movement to the appropriate part of the cell where they form the structures necessary for their normal functioning.
We can now consider the various stages of metabolism beginning with the anabolism (biosynthesis) (stages 4,5,6), leading from the carbon building blocks to complete cellular structure in a series of ATP and NAD(P)H-requiring reactions. Most of these conversions are common in all organisms. It is, however, in catabolism, which includes all the mechanisms for the generation of the central carbon intermediates and for ATP and NAD(P)H production (stages 1.2.3) that the microorganisms show much variation. We shall concentrate, therefore on the latter i.e. catabolism. (BSc Microbiology Microbial Metabolism Notes Study Material)
Anabolism
This includes the biosynthesis of monomers and coenzymes. There are a number of central metabolic intermediates which constitute the basic building blocks from which the cellular carbon is synthesized: glucose, ribose, glycerol, phosphoenol pyruvate, acetyl coenzyme-A, £-oxo-glutarate, oxaloacetate, etc. Leading from these metabolic intermediates is a series of specific pathways concerned with the biosynthesis of the monomers, coenzymes, and other essential compounds needed for growth. Some microbes have a complete set for synthesis of all organic components themselves whereas others have only restricted the ability of biosynthesis.
Autotrophs are able to assimilate inorganic carbon as CO, whereas heterotrophs get it in an organic form initially into cells. Once inside the cell, the carbon Is synthesized (maybe the reversal of catabolic processes) in its monomers. The remaining elements. N, S, H, and O are also assimilated into the cells; nitrogen as ammonia, nitrate, or molecular nitrogen (nitrogen fixation by prokaryotes only); sulphur as sulphate and hydrogen as water. Once these bio-elements are assimilated in the cell, complex organic compounds are biosynthesized.
The essential monomers must eventually be produced in an activated form so that an additional energy source is required for their polymerization. Their synthesis therefore also requires the input of energy and reducing equivalents in the form of ATP and NAD(P)H, respectively to facilitate the carbon transformation. These activated monomers are usually the same in microorganisms as in higher organisms. The methods of polymerization are similar in all organisms, differing in the type of polymer produced.
There are homopolymers (as polyglucoses, poly-B hydroxybutyrate), heteropolymers with a repeating unit i.e. regular heteropolymers (as heteropolysaccharides in the cell wall and capsule and the prokaryotic peptidoglycans) as well as heteropolymers without a repeating unit i.e, irregular heteropolymers (as those in the form of proteins and nucleic acids).
Catabolism
This concerns with the production of carbon intermediates (building blocks energy and reducing equivalents. It is important in relation to the growth and metabolism of microorganisms to consider the flux of carbon compounds in direct association with the production and utilization of ATP and NAD(P)H. (BSc Microbiology Microbial Metabolism Notes Study Material)
We have considered their interdependence in the biosynthesis of the monomeric and polymeric cellular constituents. The same is true for the pathways concerned with the provision of essential building blocks from which these cellular components are ultimately synthesized. We shall consider these pathways separately for heterotrophs and autotrophs since there are some basic differences in their metabolic pathways.
Heterotrophs
In heterotrophs, the carbon sources are catabolized by a series of reactions to provide a group of essential metabolites which are used as the basic building blocks for the synthesis of monomers, coenzymes, and fundamental structural units of the cell. Since these catabolic reactions are oxidative they also give rise to ATP and NAD(P)H. Thus, enzymes catalyzing inter-conversions such as those of the glycolytic pathway and the TCA cycle are generally common to all cellular life. They are collectively said to constitute the basal intermediary metabolism.
In heterotrophs, these enzymes are concerned both with the production of energy and reducing equivalents and with the synthesis of essential cell building blocks. However, in autotrophs where CO2 is the carbon source for growth, they usually serve an anabolic or biosynthetic role. Such enzymes and pathways which can function in both catabolism and anabolism are known as amphibolic and are usually present irrespective of the environmental conditions i.e. they are constitutive.
Many heterotrophic microbes are able to utilize different carbon sources many specific enzymes may be involved in each of the pathways. Thus a cell has the potential to produce a large number of catabolic enzymes. Such enzymes are formed only when these are required i.e. when the specific carbon source is present in the environment. These enzymes are called inducible enzymes and their synthesis requires the presence of an inducer which is usually the particular carbon course. An example is the induction of the enzyme £ -glucosidase (maltose) by the inducer maltose. It hydrolyses maltose to glucose which can be degraded by constitutive enzymes.
We have already referred to the two general phosphorylation mechanisms substrate-level phosphorylation and oxidative phosphorylation used to generate ATP by the heterotrophs during the breakdown of organic carbon and energy source. In the former, the organic substance undergoes an energy-yielding or exergonic reaction in such a way that a phosphate derivative is formed which has sufficient free energy to transfer its phosphoryl group directly to ADP to produce ATP. (BSc Microbiology Microbial Metabolism Notes Study Material)
In oxidative phosphorylation, an electron donor (AHA) is oxidized and the electrons (usually paired) so produced pass through an electron transport system to a terminal electron acceptor (B) in such a way that the energy released is partly utilized to produce ATP from ADP n, represents the number of energy-rich phosphate bonds produced in the transfer of a pair of electrons, and the value varies between 1 and 4. Besides ATP, reduced pyridine nucleotide – NAD(P)H is also produced during electron transport. The NAD(P)H produced can be re-oxidized by following three general mechanisms. (BSc Microbiology Microbial Metabolism Notes Study Material)
(1) It may donate its reducing equivalents to the electron transport chain in aerobic or anaerobic respiration, with coupled ATP synthesis.
(2) It may donate its reducing equivalents to an intermediate metabolite to give rise to a reducing fermentation product.
(3) It can be used as the source of reducing equivalents during reductive biosynthesis.
ATP – generating pathways
The heterotrophic generation of ATP involves the conversion of an organic substrate molecule to end products via a metabolic pathway that has sufficient free energy so that it can be coupled with ATP synthesis. This process involves the breakdown of an organic molecule to smaller molecules and is called catabolism. In such a pathway, oxidation reactions provide sufficient free energy to drive the conversion of ADP to ATP. These oxidation reactions are coupled with a simultaneous reduction reaction that balances the transfer of electrons.
Fermentation versus Respiration
Heterotrophs exhibit two basic strategies, fermentation and respiration for oxidizing organic compounds to synthesize ATP from ADP. In fermentation, the organic substrate acts as an electron donor (reducing agent) and a product of that substrate acts also as an electron acceptor (oxidizing agent). Therefore both, the electron donor and the acceptor are internal to the organic substrate. There is no net change in the oxidation state of the products relative to the starting substrate molecule.
The oxidized products are exactly counterbalanced by the reduced products, and thus the required oxidation-reduction balance is achieved. The coenzymes, that are reduced are reoxidized by its end so that they are infact not consumed in the process. There is no requirement for oxygen or other electron acceptors. (BSc Microbiology Microbial Metabolism Notes Study Material)
In contrast to fermentation, respiration requires an external electron acceptor; that is a molecule other than the one derived from the electron donor must act as an electron acceptor (oxidizing agent) to achieve a balance of oxidation-reduction reactions. This balance of O-R is thus also achieved without the consumption of co-enzymes. The most common external electron acceptor in respiration is molecular oxygen – thus called aerobic respiration. When another molecule as nitrate or sulphate, serves as the terminal electron acceptor, the pathway is called anaerobic respiration. (BSc Microbiology Microbial Metabolism Notes Study Material)
Fermentation yields far less ATP per substrate molecule than respiration since the same substrate serves both donor and acceptor of electrons. There is no complete oxidation. The ΔGo for the complete oxidation of glucose to carbon dioxide and water is 686 kcal/mole, compared to only 58 kcal/mole when glucose is partially oxidized to two molecules of lactic acid in fermentation.
Depending upon the conditions of growth, an organism uses either the respiration or fermentation catabolic pathways (i.e. respiration – anaerobic or aerobic OR fermentation). Therefore three general methods exist by which a carbon and energy source can be broken down to provide energy. (BSc Microbiology Microbial Metabolism Notes Study Material)
Aerobic respiration. Respiration can be defined as an ATP-generating metabolic process in which either organic or inorganic compounds serve as electron donors (become oxidized) and inorganic compounds serve as the ultimate acceptors (become reduced). Usually, the ultimate electron acceptor is molecular oxygen and this is called aerobic respiration. Thus carbon and energy source is broken down by a series of reactions, the oxidation stages occurring at the expense termed respiration. of oxygen as the terminal electron acceptor. Aerobic respiration is also simply termed respiration. (BSc Microbiology Microbial Metabolism Notes Study Material)
In aerobic respiration, sugars are first converted to the key metabolic intermediate, pyruvic acid. Certain catabolic reaction pathways are common to both respiration and fermentation. Among these are the three pathways of conversion of sugars to pyruvic acid.
They are: (i) the Embden-Meyerhof pathway (also called the glycolytic pathway), (ii) the pentose phosphate pathway also called the hexose monophosphate shunt, and (iii) the Entner-Doudoroff pathway. The first two occur in many organisms (pro-as well as eukaryotes), whereas the third is restricted to some prokaryotes. We shall consider the glycolytic pathway, which lost common, not only in microorganisms but also in plants and animals.
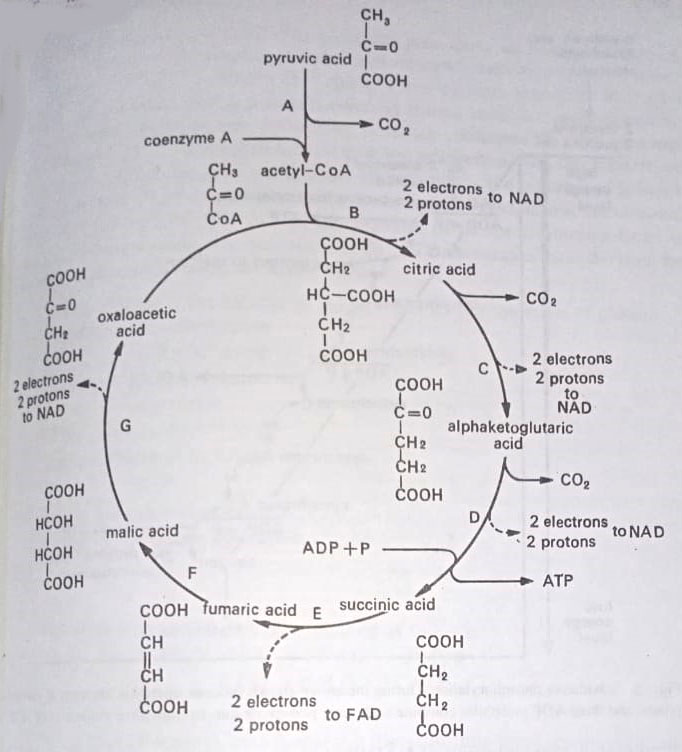
Glucose is first converted to pyruvic acid through glycolysis, and this acid is then oxidized to CO2 through the TCA or Krebs’ cycle.
The glycolytic pathway is the best known, which was discovered by Gustav Embden and Otto Meyerhof in the 1930s. This can occur in absence of oxygen and involves nine chemical steps, each step catalyzed by a specific enzyme. The end result of glycolysis is to split a 6-carbon glucose molecule into two 3-carbon pyruvic acid molecules. In glycolysis, there are produced two molecules of pyruvic acid, two molecules of NAD(P)H, and two molecules of ATP, generated by substrate-level phosphorylation.
i,e. C6H12O6 + 2 NAD++ 2 ADP + 2 Pi → 2 CH3CO.COOH + 2NADH + 2 H+ + 2 ATP
For the reaction to continue NAD(P)H must by reoxidized.
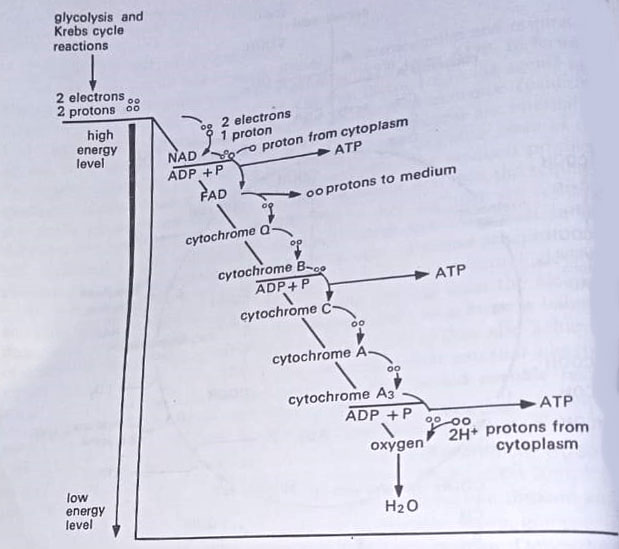
The pyruvic acid is then oxidized through Krebs’ cycle, named for Hans A. Krebs who won the Nobel Prize in 1953 for his discovery of several of the intermediates. The Krebs’ cycle utilizes the pyruvic acid generated in glycolysis. Krebs’ cycle is the major part of the ATP being formed by oxidative phosphorylation. The direct gain in Kerbs’ cycle is 2 molecules of ATP (one for each molecule of pyruvic acid). The direct gain in glycolysis is also 2 molecules of ATP.
Like glycolysis, there are, however, several points in Krebs’ cycle at which oxidative phosphorylation takes place. It is here that a major part of the energy is captured in the form of ATP. The electrons pass along an electron transport chain, a series of chemical molecules, and descend step by step to a lower energy level. Within this chain, some carriers transport hydrogen atoms (an electron plus a proton), whereas others transport only electrons. The transfer of electrons, from the reduced coenzyme to the terminal electron acceptor, establishes a hydrogen ion gradient across the membrane that is critical for ATP generation.
In eukaryotes, the membrane is the inner mitochondrial membrane, whereas in bacteria it is a cytoplasmic membrane. This process of ATP is called chemiosmosis. To summarise, 28 ATP molecules are derived by oxidative phosphorylation in Krebs’ cycle.
The following is the balance of ATP molecules per molecule of glucose:
Oxidative
phosphorylation in Krebs’ cycle – 28
Oxidative
phosphorylation in glycolysis – 6
Direct gain (substrate-level
phosphorylation) in Krebs’ cycle – 2
Direct gain (substrate-level
phosphorylation) in glycolysis – 2
Total – 38
The overall oxidation can be explained as follows:
C6H12O6 + 6 O2 + 38 ADP + 38 P → 6 CO2 + 6 H2O + 38 ATP
Of the 38 ATP formed, only four resulted from substrate-level phosphorylation.
A single molecule of glucose has approximately 690,000 calories of energy. In this system, 380,000 calories are captured in 38 molecules of ATP. Each ATP molecule traps 10,000 calories in the bonding of ADP to phosphate ions. Thus in this system, approximately 55 percent of the chemical energy is thus preserved. (BSc Microbiology Microbial Metabolism Notes Study Material)
Anaerobic respiration. Although oxygen is the most common and efficient electron acceptor, some prokaryotes are able to utilize certain inorganic compounds as alternative terminal electron acceptors in a process called anaerobic respiration. The compounds that act as electron acceptors are nitrates, sulfates, and carbonates. They are strict anaerobes and can not use aerobic respiration as alternative means of generating ATP. Thus, nitrate is reduced to nitrite, nitrous oxide, or molecular nitrogen, and sulphate is reduced to sulphide.
In a parallel series of reactions in methanogenic bacteria, Methanobacterium, and Methanococcus, CO2 is used as an electron acceptor and reduced to methane during the oxidation of hydrogen (the mechanism, however, does not involve a conventional electron transport chain).
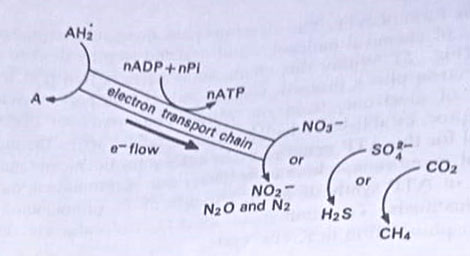
Species of Serratia and Pseudomonas use nitrate ions (NO–3) as electron acceptors and the ions are converted to nitrite (NO–2) ions. There are other denitrifiers that reduce nitrates to nitrous oxide and nitrogen. Members of the genus Desulfovibrio use sulphate ions (SO2=) as electron acceptors converting them to S or H2S. Clostridium aceticum uses CO2, converting it to acetic acid during the oxidation of hydrogen.
However, the amount of ATP formed in anaerobic respiration is less than in aerobic respiration. This is because electron acceptors in anaerobic respiration have lower redox potentials than oxygen. Anaerobic respiration actually plays an important part in maintaining the cycles of elements in nature. (BSc Microbiology Microbial Metabolism Notes Study Material)
Fermentation. It can be defined as “an ATP-generating metabolic process in which organic compounds serve both as electron donors (becoming oxidized) and electron acceptors (becoming reduced)”. The compounds that perform these two functions are usually two different metabolites derived from a single fermentable (usually an intermediate compound in the process of respiration) substrate. The average oxidation level of the end products is identical to that of the substrate. Thus fermentation must balance. Pasteur defined it as “Life without air”.
Unlike respiration, oxidative phosphorylation and chemiosmotic generation of ATP do not occur in fermentation. In fermentation, no external electron acceptor is required. Thus carbon and energy source is broken down by a series of reactions that produce ATP by substrate-level phosphorylation. Since fermentations are balanced ((balance between oxidation and reduction), pyridine nucleotides reduced in one step of the process are subsequently oxidized in another. (BSc Microbiology Microbial Metabolism Notes Study Material)
Because they do not require oxygen, all fermentation pathways are anaerobic, and microbes that generate their energy by fermentation are carrying out anaerobic metabolism, regardless of whether or not the organism is growing in the presence of molecular oxygen.
Fermentation pathways
The initial steps are identical to those of respiration. For example, for carbohydrate fermentation, the pathway begins with glycolysis. In EM glycolytic pathway, there are generated two pyruvate molecules, two reduced coenzyme NADH molecules, and two ATP molecules for each molecule of glucose. The remainder of the fermentation pathway is concerned with reoxidizing the coenzyme.
In fermentation, reoxidation of NADH to NAD+ depends on the reduction of pyruvate molecules formed during glycolysis. Different microorganisms have developed different pathways for utilizing the pyruvate for reoxidizing the reduced coenzyme with different terminal sequences of the various fermentation pathways resulting in the formation of various end products. The different fermentation pathways are named for the characteristic end products that are formed. The most common ones are as follows:
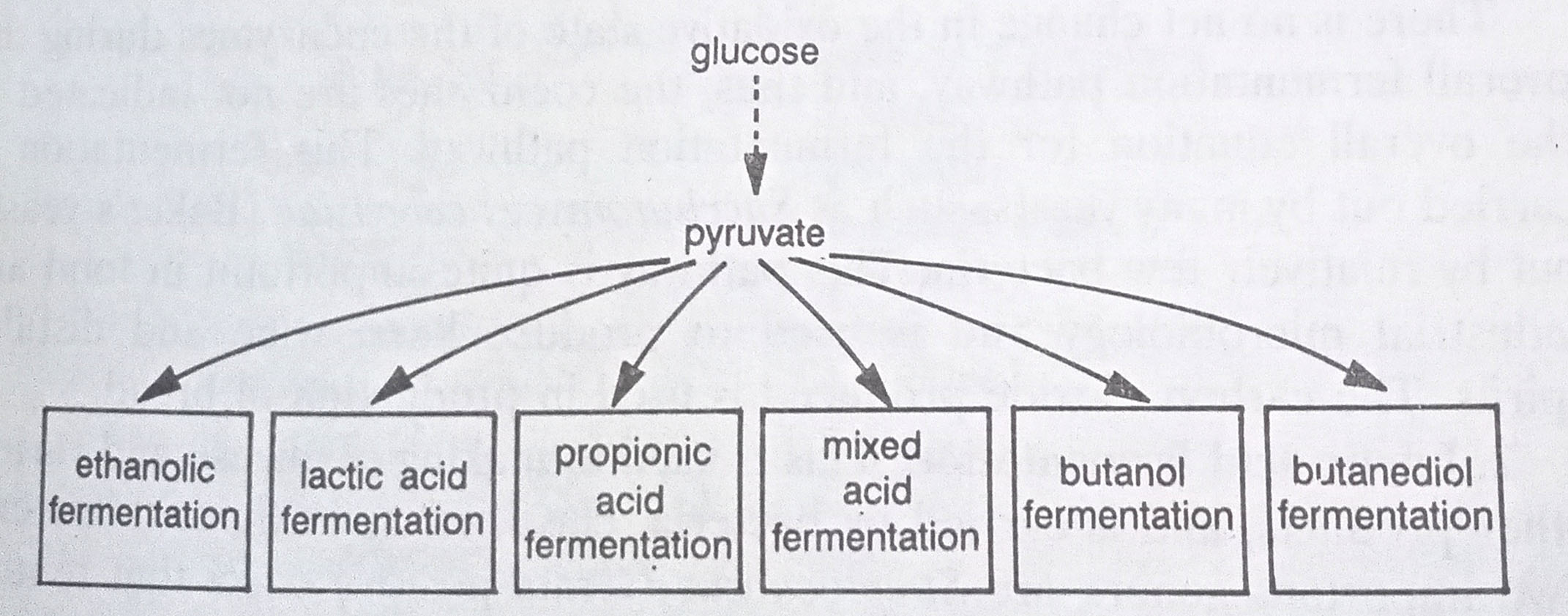
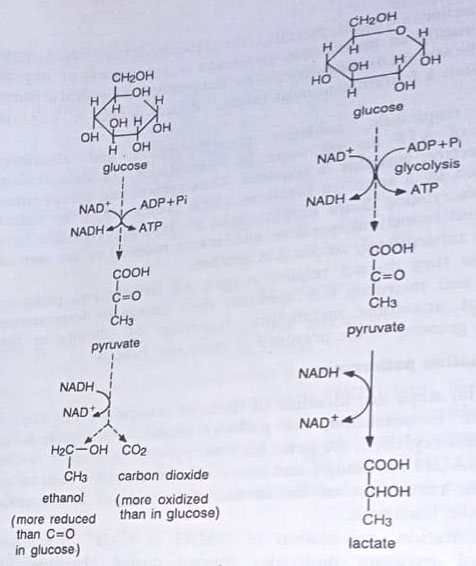
Ethanolic fermentation. Ethanol is the end product. Here pyruvate is converted to ethanol and carbon dioxide. When glucose is the substrate and the EM pathway is followed, the equation for this fermentation is:
Glucose + 2ADP + 2Pi → 2 Ethanol + 2CO2 + 2ATP
There is no net change in the oxidative state of the coenzymes during the overall fermentation pathway, and thus, the coenzymes are not indicated in the overall equation for the fermentation pathway. This fermentation is carried out by many yeasts, such as Saccharomyces cerevisiae (Baker’s yeast), but by relatively few bacteria. This pathway is quite important in food and industrial microbiology and is used to produce beer, wine, and distilled spirits. The carbon dioxide produced is used in the production of bread.
Lactic acid fermentation. This is used in the making of cheese and various other products and is carried by bacteria, classified as lactic acid bacteria. Two important genera are Streptococcus (Gram-positive cocci that tend to form chains) and Lactobacillus (Gram-positive rods that tend to form chains). In this pathway, pyruvate is reduced to lactic acid with the coupled reoxidation of NADH to NAD+. The overall pathway can be expressed as follows:
Glucose + 2ADP + 2Pi → 2 Lactic acid + 2ATP
When the EM pathway of glycolysis is used in lactic acid fermentation, the overall pathway is called a homolactic fermentation, because the only end product formed is lactic acid. Homolactic fermentation is carried out by Streptococcus, Pediococcus, and various Lactobacillus species. This pathway is quite important in the dairy industry.
This pathway is used for souring milk and also for the production of various types of cheese, yogurt, and other dairy products. Streptococci living on tooth surfaces produce lactic acid. Lactobacilli occur in the human digestive tract and help in the digestion of milk. Lactobacillus acidophilus is added to the milk diet of those unable to digest milk carbohydrates.
In contrast to the homolactic acid pathway, some microbes carry out a heterotactic acid fermentation, using the pentose phosphate pathway rather than the EM pathway of glycolysis. This is so named because ethanol and carbon dioxide are also produced in addition to lactic acid. The overall reaction can be expressed as follows:
Glucose + ADP + Pi → Lactic acid + Ethanol + CO2 + ATP
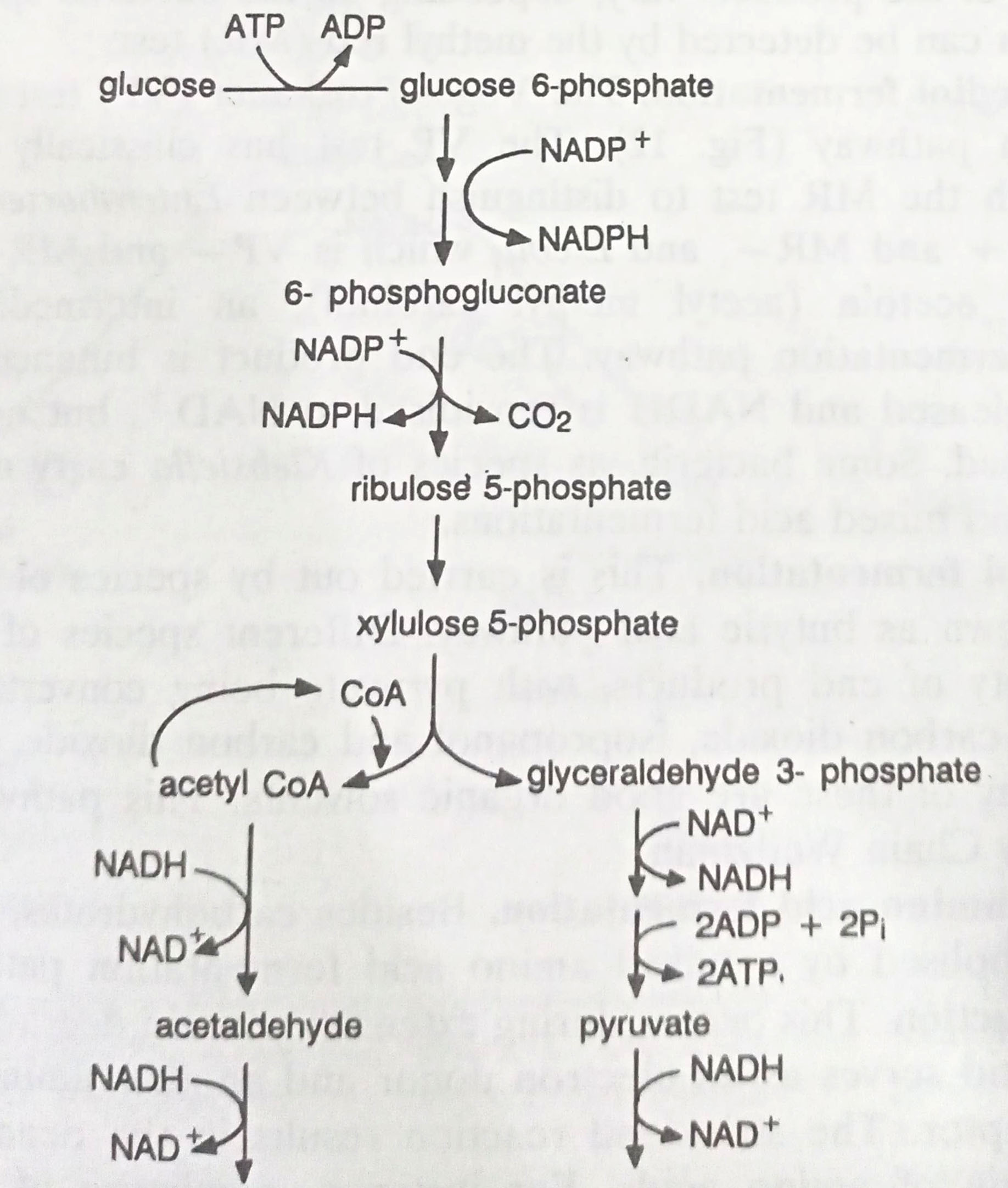
This pathway is carried out by Leuconostoc and various Lactobacillus species. Leuconostoc spp. are used in production of sauerkraut.
Propionic acid fermentation. The end product is propionic acid, This is carried out by the bacteria that are able to carry out a fermentation beginning with lactic acid as the substrate. Lactic acid bacteria convert the initial substrate in the milk to lactic acid and species of Propionibacterium, Gram-positive rods subsequently convert this lactic acid to propionic acid and carbon dioxide. Only after the cheese curd has formed by lactic acid bacteria, do the propionic acid bacteria begin their fermentation. Gas bubbles in the semisolid cheese curd are due to carbon dioxide, produced during this late fermentation. (BSc Microbiology Microbial Metabolism Notes Study Material)
Mixed acid fermentation. Whereas propionic acid fermentation is carried out by a few species of bacteria, the mixed acid fermentation pathway is relatively common. This is named so because end products formed are a mixture of products. It is carried out by members of Enterobacteriaceae, a large family that includes also E.coli. The pyruvate formed during glycolysis is converted to a variety of products, including ethanol acetate, formate, molecular hydrogen, and carbon dioxide. The proportions of the products vary, depending on the bacterial species. Such fermentation can be detected by the methyl red (MR) test.
Butanediol fermentation. The Voges-Proskauer (VP) test detects the fermentation pathway. The VP test has classically been together with the MR test to distinguish between Enterobacter aerogenes, which is VP+ and MR-, and E.coli, which is VP – and MR +. The VP test detects acetoin (acetyl methyl carbinol), an intermediate in the butanediol fermentation pathway. The end product is butanediol, carbon dioxide is released and NADH is reoxidized to NAD+, but no additional ATP is formed. Some bacteria, such as species of Klebsiella, carry out both butanediol and mixed acid fermentation.
Butanol fermentation. This is carried out by species of Clostridium, also known as a butyric acid pathway. Different species of Clostridium form a variety of end products, with pyruvate being converted to either acetone and carbon dioxide, isopropanol and carbon dioxide, butyrate, or butanol. Many of these are good organic solvents. This pathway was first discovered by Chain Weitzman.
Mixed amino acid fermentation. Besides carbohydrates, amino acids can be metabolized by a mixed amino acid fermentation pathway – the Strickland reaction. This occurs during extensive protein degradation, where one amino acid serves as an electron donor and another amino acid as an electron acceptor. The Strickland reaction results in the deamination and decarboxylation of amino acids.
For instance, a mixture of alanine and glycine can yield the end products acetate, carbon dioxide, and ammonia. This fermentation is responsible for the pleasant odours of some wines and cheeses, but also responsible in part for the horrible smell of a gangrenous wound.
Autotrophs
In these microbes, CO2 is the source of carbon. It is converted into the basal intermediary metabolic system by a series of reactions – the Calvin cycle which appears to be common to all such organisms i.e. to the photoautotrophs or chemoautotrophs. Unlike the oxidative catabolism of heterotrophs, however, the Calvin cycle is reductive and requires an input of reducing power and energy in the form of NAD(P)H and ATP respectively. (BSc Microbiology Microbial Metabolism Notes Study Material)
Autotrophs belong to the following two groups according to their mode of energy production:
[I] Photoautotrophs (Photolithotrophs)
They derive energy by photophosphorylation, a process involving the excitation of chlorophyll molecules by light with the consequent emission of electrons which are transferred in a reductive reaction to an acceptor of low redox potential. This primary acceptor can then be reoxidized by the transfer of the electrons through an electron-transport system with concomitant production of ATP by a process analogous to that of oxidative phosphorylation. (BSc Microbiology Microbial Metabolism Notes Study Material)
Although the basic chemiosmotic mechanism driving the synthesis of ATP during photophosphorylation is the same in all photoautotrophs, there is a major difference between algae and anaerobic photosynthetic bacteria in respect of the photosystems they employ to transfer energy absorbed from light irradiation to ATP synthesis. (BSc Microbiology Microbial Metabolism Notes Study Material)
In the case of anaerobic green and purple photosynthetic sulphur bacteria, there is only one photosystem, known as photosystem I or cyclic photophosphorylation. The cyanobacteria and the algae have two photosystems, referred to as photosystem I and photosystem II or cyclic and non-cyclic photophosphorylation. (BSc Microbiology Microbial Metabolism Notes Study Material)
The Z pathway of photophosphorylation.
Photosystems I and II are normally linked in a Z pathway of photophosphorylation in cyanobacteria and algae. This is also known as oxygenic (oxygen-evolving) photophosphorylation and is also characteristic of plants. The combined photosystems require two separate photo-acts, that is, the absorption of light energy at two different photoactivation centers. In algae and cyanobacteria, the primary photo-synthetic pigment is chlorophyll. Light energy absorbed by accessory pigments is also transferred to chlorophylla, which absorbs light at a wavelength of 700 nm (photosystem I), and therefore designated as a P700 molecule.
In photosystem II the photoreceptor is designated P680, representing a molecule of chlorophyll that has been modified to absorb light at a wavelength of 680 nm. When the PS II center absorbs light energy, it becomes excited and emits an electron initiating a non-cyclic pathway in which the electron is transferred through a series of membrane-bound electron carriers (transport chain). In the combined Z pathway the electrons from PS II are transferred through a series of electron carriers to the P700 chlorophyll reaction center molecule of PS I.
This transfer of an electron from an excited PS II to PS I establishes a sufficient hydrogen ion gradient across the membrane to drive ATP synthesis. The electron transport chain is continued when a molecule of P700 chlorophyll absorbs light energy, initiating the electron transfer sequence of PS I. The electron that is transferred from PS II is used to balance the oxidation state of the P700 chlorophyll molecule. The electrons transferred through PS I normally are used to reducing the coenzyme NADP+ to NADPH, the reducing power of biosynthesis.
The unidirectional (non-cyclic) flow of electrons to reduce NADP+ to NADPH leaves a charged P680 chlorophyll molecule that must be reduced to balance the oxidation-reduction state of this pathway. In order to accomplish this needed balance of reaction, a water molecule is split light (photolysis of water, an enzyme-independent reaction, unusual in biosynthetic pathways) forming oxygen and transferring electrons to P680 chlorophyll of PS II. It also appears that a second ATP molecule may be generated as a consequence of electron transport from water to PS II, creating a hydrogen ion gradient across the membrane.
Anoxygenic photosynthesis. Although the movement of electrons through the entire Z pathway is normally noncyclic, with the electron flow from an electron donor, water to coenzyme, NADP+, electrons may also take a cyclic route through PS I. When this occurs NADPH is not generated, but ATP is synthesized, that is PS I can act as a cyclic photophosphorylation system, providing the organism with an increased yield of ATP. At low light intensities, the cyanobacteria can carry out anoxygenic photosynthesis (non-oxygen evolving photosynthesis), during which PS I follows a cyclic photophosphorylation pathway.
In the cyclic route, PS II is inoperative and thus there is no photolysis of water to yield oxygen. In the cyclic route, the electrons lost from PS I fall down and return back to it via the electron transport chain used by electrons of PS II. While carrying out oxygenic photosynthesis, cyanobacteria derive their reducing power NADPH from the coupled oxidation of H2S with coenzyme reduction. During utilization of H2S, granules of elemental sulphur thus formed become deposited outside of the cells of cyanobacteria.
The process of anoxygenic photosynthesis carried out by photoautotrophic bacteria other than cyanobacteria is similar in many ways to cyanobacterial photosynthesis. The anaerobic photosynthetic bacteria (green and purple sulphur bacteria) only carry out the reactions of PS I. In these bacteria, the primary pigment is bacterial chlorophyll which absorbs light energy of longer (870 nm) wavelengths than does chlorophyll a. As with cyanobacteria, algae, and plants, light energy absorbed by accessory pigments is also transferred to this pigment. The excited P870 emits an electron that moves through the electron transport chain.
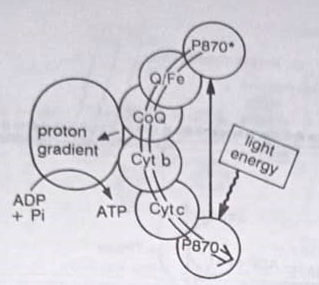
Instead of using any external donor to reduce the oxidized chlorophyll molecule, the electron is transferred back to the chlorophyll molecule in a cyclic manner. Thus chlorophyll molecule acts as an internal electron donor and acceptor for PS I. During the transfer of an excited electron to the oxidized chlorophyll molecule, one ATP is synthesized. Cyclic photophosphorylation generates ATP without generating reduced coenzyme, NADPH. These bacteria generate NADPH by utilizing reduced compounds, such as H2S, as electron donors.
The flow of electrons from such external donor to NADPH may pass through PS I in a noncyclic manner. However, in order to generate NADPH directly from H2S or other sulphur compounds, insufficient energy is released from these compounds. Although the reaction is light-dependent, reduced sulphur compounds can donate electrons only at a redox level more oxidized than NADP. Reduced NADP can only be thus generated by energy (ATP)-driven reversed flow of electrons.
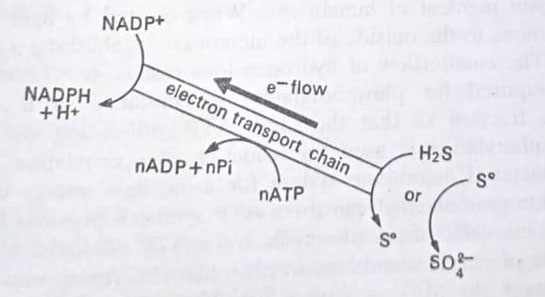
Such reverse electron flow is necessary for the metabolism of some chemoautotrophs also, such as those oxidizing Fe+2 or NO2+– because the direct generation of reducing power by transfer of electrons from the inorganic substrate to NAD+ is thermodynamically unfavorable.
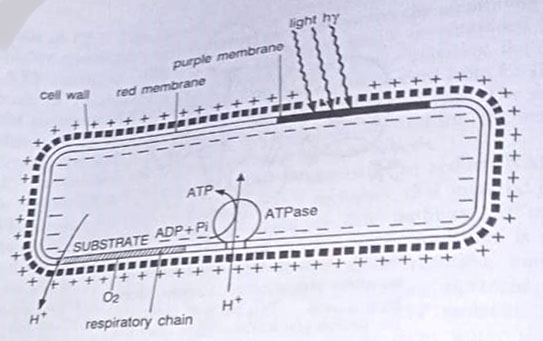
The purple membrane of halobacteria. Halobacterium, an archaebacterium can generate ATP both by using an organic substrate and by using light energy. In presence of oxygen, these bacteria use oxidative phosphorylation for ATP synthesis (aerobic respiration). The electron transport chain for this pathway is located in a portion of the cytoplasmic membrane that is red in colour and hence called the red membrane. (BSc Microbiology Microbial Metabolism Notes Study Material)
In the absence of oxygen, they turn to photophosphorylation for ATP synthesis. The mechanism here is different from other bacteria. It is based upon a purple membrane portion of the cytoplasmic membrane that contains bacteriorhodopsin, a protein of a chemical structure similar to that of the rhodopsin pigment of the human eye. When excited by light this pigment pumps protons to the outside of the membrane establishing a hydrogen ion gradient. The counterflow of hydrogen ions results in ATP synthesis.
The enzyme required for phosphorylation is contained in a separate red membrane fraction so that the same ATP-synthesising system used for photophosphorylation is used for oxidative phosphorylation (respiration). The halobacteria membrane system for using light energy to establish a hydrogen ion gradient that can drive ATP synthesis provides firm evidence for the essential role of chemiosmosis in ATP synthesis.
The role of the purple membrane in photophosphorylation was accomplished largely through the work of Walter Stoeckenius and co-workers. Through experimental work, they could show that Halobacterium can carry out both, aerobic respiration and light-coupled photophosphorylation of ATP synthesis. (BSc Microbiology Microbial Metabolism Notes Study Material)
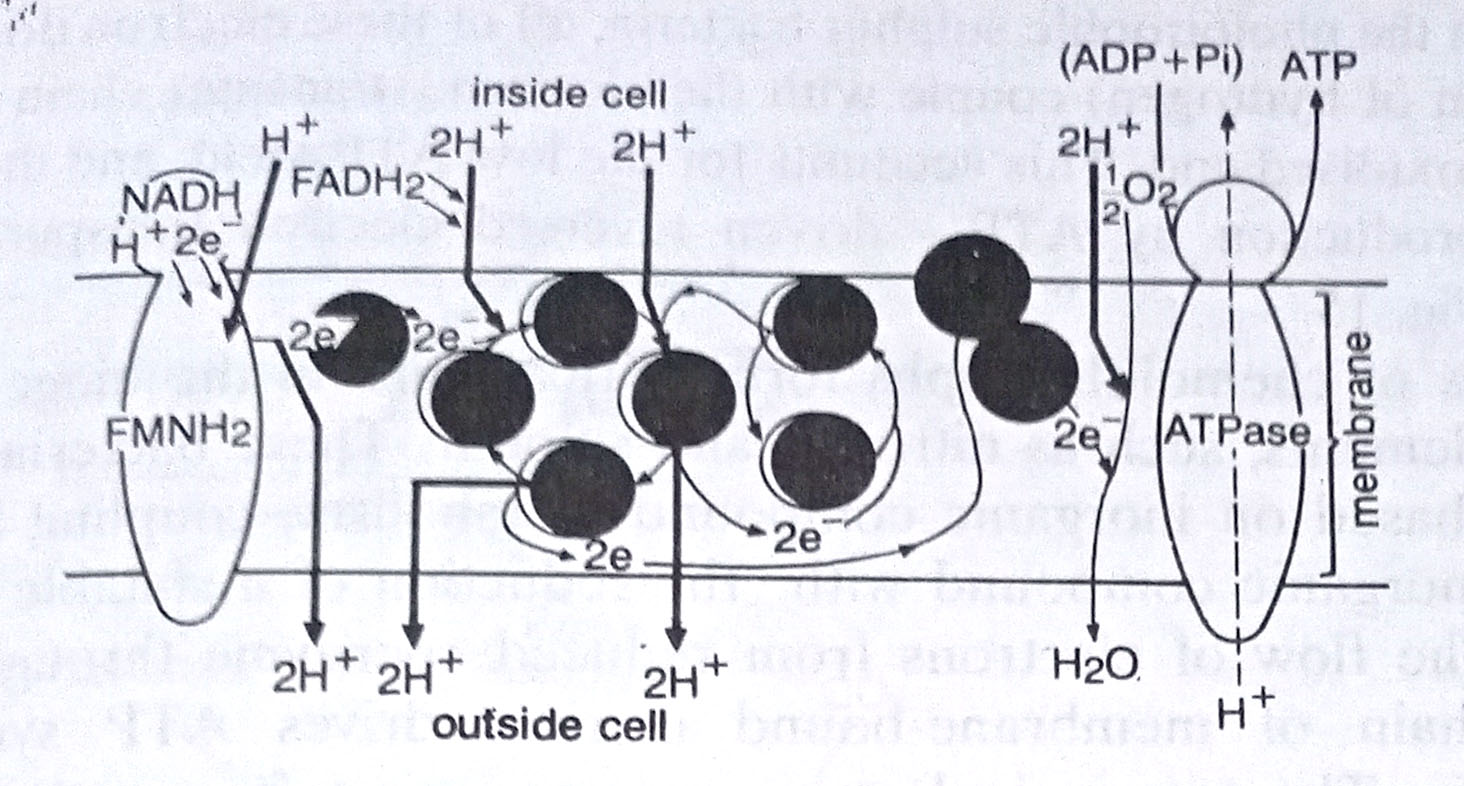
These observations were consistent with the hypothesis that respiration and light generate an electrochemical proton gradient across the cell membrane and this gradient drives the ATP synthesis by membrane ATPase. These workers made artificial vesicles from the purple membrane and designed experiments to establish the role of bacteriorhodopsin in such a process. This work was chiefly done by Stoeckenius and Efraim Racker. (BSc Microbiology Microbial Metabolism Notes Study Material)
[II] Chemoautotrophs (Chemolithotrophs)
These bacteria obtain their energy by the oxidation of inorganic substrates, usually at the expense of oxygen as the terminal electron acceptor (i.e. generally aerobic). ATP is formed by oxidative phosphorylation and the basic difference from energy production in aerobic heterotrophs is that the electron donor happens to be an inorganic compound (in aerobic heterotrophs, it is an organic compound).
The chemoautotrophs are an important group from an economic point of View. One of them has also become something of a cause Celebre. Thiobacillus thioxidans gets its carbon from CO2, and its energy by oxidizing inorganic sulfur compounds to sulphuric acid, it will grow at very acidic, PH and it can not grow normal organic substrates such as glucose which may actually be inhibitory.
But the intermediary metabolism of this organism is little different from that of any other autotroph which uses CO2 as the sole carbon source.
As with the phototrophic sulfur bacteria, all of these electron donors (with the exception of hydrogen) coupled with the electron–transport chain at a point close to the oxidized end. This accounts for the low ATP yield, and the need for NAD(P)H production by ATP – driven reversed electron transport in these organisms. (BSc Microbiology Microbial Metabolism Notes Study Material)
Activities of chemolithotrophs form critical links in the biogeochemical cycling of elements, such as nitrogen and sulphur. These bacteria carry out respiration based on inorganic compound metabolism, coupling the oxidation of an inorganic compound with the reduction of a suitable coenzyme. The flow of electrons from reduced coenzymes through the electron transport chain of membrane-bound carriers drives ATP synthesis by chemiosmosis. The terminal electron acceptor most frequently is oxygen, though some are able to carry out anaerobic respiration.
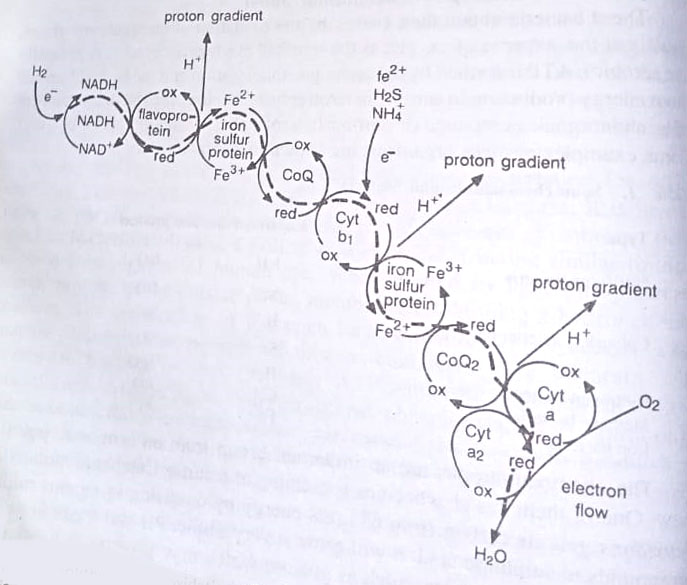
Examples of some chemolithotrophs are given in Table. It may be seen that there are hydrogen bacteria that produce the enzyme hydrogenase that is used in the metabolism of molecular hydrogen, forming water and reduced coenzyme NADH. The oxidation of reduced compounds containing either iron, sulphur or nitrogen, however, follows a different route in these bacteria.
Some of the electrons flow toward the terminal electron acceptor, oxygen via the electron-transport chain whereas other electrons flow in the reverse direction to produce reduced coenzyme needed for biosynthesis. Consequently for their growth and reproduction, these bacteria must oxidize very large amounts of reduced nitrogen, sulphur, or iron-containing compounds. Some examples are explained briefly below.
Sulphur oxidation. The most common compounds used are H2S, elemental sulphur, and thiosulphate. Such bacteria may be important producers in some marine systems. Elemental sulphur becomes deposited by the bacteria, which use H2S as the substrate, within or outside their cells.
Some sulphur oxidizing forms as Thiobacillus thiooxidans can utilize large amounts of reduced sulphur compounds, forming sulphates. This bacterium is important due to its involvement in the formation of acid mine drainage problems and its use in mineral recovery processes. T. ferrooxidans which oxidises both reduced sulphur and reduced iron for ATP generation, often is found in acid mine drainage streams, where it has an available source of reduced sulphur and reduced iron that it can use for ATP generation.
Nitrification. Nitrifying bacteria oxidize either ammonium or nitrite ions. Nitrosomonas oxidises NH4+ to NO2–. Nitrobacter then oxidizes NO2– to NO3–. Again as this yield very low ATP, these microbes carry out other transformations in soil for the synthesis of required ATP. These bacteria have a marked influence on soil fertility as NH4+ ions bind to negatively charged soil clay particles, whereas the negatively charged NO2– and NO3– do not bind and are leached from the soil by rainwater.
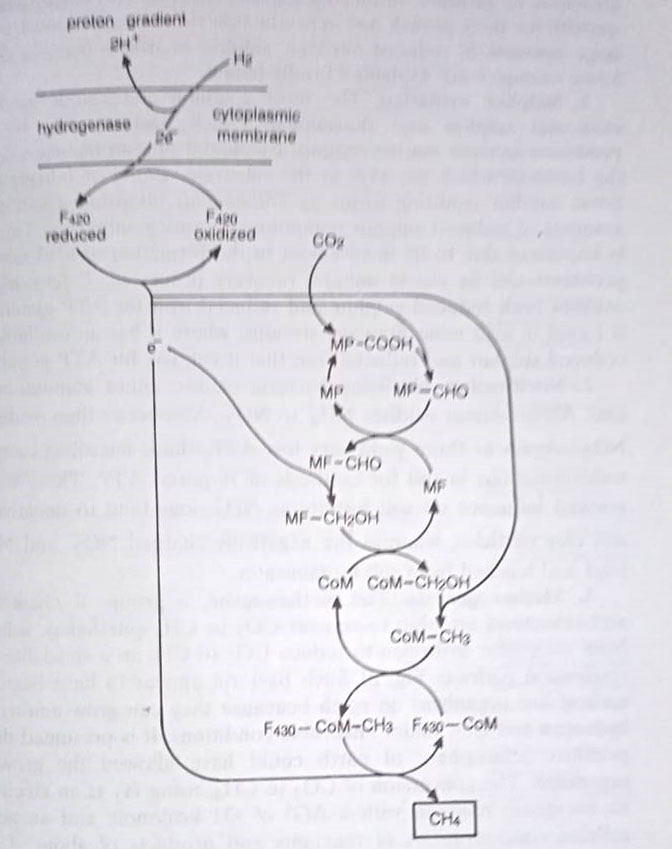
Methanogenesis. The methanogens, a group of chemolithotrophic archaebacteria are able to convert CO2 to CH4 (methane), using electrons from molecular hydrogen to reduce CO2 to CH4 in a specialized anaerobic respiration pathway. Such bacteria appear to have been among the earliest live organisms on earth because they can grow autotrophically on hydrogen and CO2 under anaerobic conditions. It is presumed that the early primitive atmosphere of the earth could have allowed the growth of such organisms.
The conversion of CO2 to CH4, using H2 as an electron donor is an exergonic reaction with a ΔGo of -31 kcal/mole and an actual ΔG at cellular concentrations of reactants and products of about -15 kcal/mole. ATP synthesis is an oxidative phosphorylation way rather than direct substrate-level phosphorylation. Several cofactors unique to this system have been identified.
For every molecule of CO2 converted to CH4 one molecule of ATP is generated, along with NADPH. This NADPH is used for the incorporation of CO2 into the macromolecules of the cell. Approximately 90-95% of CO2 used by methanogens is converted to CH4.
BSc Microbiology Microbial Metabolism Notes Study Material
BSc 2nd Year Sample Model Practice Mock Test Question Answer Papers