Bacteriophage (Bacterial Virus)
They, also commonly known as phages attack bacterial cells. Phages were discovered in 1915 by Twort, and d’ Herelle (1917) studied them in detail. The morphology of phages varies from exceedingly simple such as the small, single-stranded DNA phage, Φ x 174, to phages with a complex tadpole-like shape, the T-even phage. A number of phages are known to attack the cells of the bacterium, Escherichia coli, and the many varieties of phages as described by Bradley (1967) are shown in Figure.
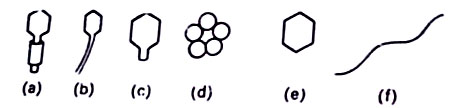
The major properties of these phage types are listed in Table.

Ultrastructure of phage
As shown in Figures T-even coliphage is a tadpole-like structure with a head and a tail. The head is in the form of a bipyramidal, hexagonal prism, whereas the tail is cylindrical. The tail is attached to one end of the head. The head consists of a proteinaceous membrane enclosing a core of viral DNA. DNA is a single, thread-like, double-stranded macromolecule, about 50 m long.
The tail is made up of four components. It has (i) a central helical hollow tube or core (through which the viral DNA passes during the infection of the host cell). (ii) the core is surrounded by a helical proteinaceous sheath, which is capable of longitudinal contraction. The sheath is connected to a thin disc or collar at the head end. (iii) at the distal end the sheath is connected to a hexagonal basal plate, which is of complex structure. The plate has a pin at every corner. (iv) the plate along with its pins is connected to six long thin tail fibers which are the organs of attachment to the wall of the host cell.
We shall now describe in some detail the different steps of the life cycle of a T-even phage which follows a lytic type of cycle in its bacterial host cell. The main steps are, (i) infection of the host cell, (ii) viral multiplication, and (iii) maturation of viral particles and their release after the lysis of the host cell.
Infection of host cell
The first step in infection is a highly specific interaction of the phage’s adsorption organelle, such as the tail, with receptors on the surface of the host cell, which leads to the attachment of the phage to the cell adsorption. Then the DNA is released from the capsid and enters the cell.

- Adsorption. The initially reversible attachment of the phage to the receptors rapidly becomes irreversible (i.e. the phage cannot be washed away). All virions have a specialized structure for adsorption. In the T-even coliphages, it is a complex structure: the tail fiber. The tips of the fibers attach first and reversibly to the cell surface and are followed by the tail pins which attach irreversibly.
The adsorbed phage acquires its head perpendicular to the cell wall. The host cell receptors are often complex polysaccharides with phage-binding and antigenic specificity. With capsulated host cells, an enzyme in the tip of the phage tail hydrolyses specific capsular polysaccharides, digging a tunnel through which the phage reaches the cell wall. The receptor for phage is a mannose transport protein. Some male-specific coliphages adsorb only to the sex pili of F+ cells. Phage f2 (RNA-containing) adsorbs exclusively on its tip.
2. Separation of nucleic acid from the coat. Details were given by Hershey and Chase (1952), showing that phage DNA carried the genetic information of the phage into the cell. Injection of DNA into the cell does not require energy from the cell. After the DNA is injected, the intact cells can produce plaques, but disrupted cells cannot. However, infectivity reappears later when the progeny virus is formed.
The temporary disappearance of infectivity, called eclipse, is due to the inability of the naked viral DNA to infect bacteria under ordinary conditions. (BSc 2nd Year Microbiology Viruses Notes Study Material)
3. Mechanism of penetration of the nucleic acid. Nucleic acid, generally associated with some internal proteins of the virion, is released from the capsid after the virion has become irreversibly adsorbed. It penetrates into the cells, apparently at sites at which the outer and inner membranes are in contact, and remains associated with the membrane. DNA released from virion is protected from membrane nucleases by the associated proteins and by DNA modifications. (BSc 2nd Year Microbiology Viruses Notes Study Material)
The T-even phages have a highly specialized mechanism for releasing their DNA Electron microscopy revealed that after the tip of the tail has become anchored to the cell surface, the contraction of the tail sheath pulls the collar and the phage head toward the plate, pushing the tube through the cell wall. When the tube reaches the plasma membrane, the DNA is ejected. Because of this action, as well as its shape, the virion has been likened to a hypodermic syringe, and the release of the nucleic acid is called an injection.
Contraction is the result of a chain of conformational changes initiated by the attachment of fibers and pins to the cell. The hexagonal base plate becomes starlike and separates from the tube; then the sheath shortens and thickens. Bacteria can also be infected by purified phage DNA after pretreatment with Ca2+ or conversion to spheroplasts. However, the efficiency of this process, called transfection, is very low, because the DNA is likely to be degraded by exonucleases. (BSc 2nd Year Microbiology Viruses Notes Study Material)
4. Effect of phage attachment on cellular metabolism. The attachment of the T-even phage tail per se, without expression of viral genes, causes disorganization of the cell plasma membrane. This causes profound metabolic changes, including an almost immediate cessation of cellular protein synthesis. These changes lead to cell lysis without viral multiplication (lysis from without).
In infected cells, the membrane alterations and their metabolic effects are only transient and are rapidly reversed by the incorporation of several page-specified proteins in the membrane. These proteins cause other effects: make the cell resistant to superinfection by phage of the same type and to lysis from without by superinfecting ghosts, and they cause lysis inhibition.
Viral Multiplication Cycle
The process of viral multiplication, initiated by the penetration of the patent nucleic acid, involves many sequential steps, which end in the release of the newly synthesized progeny virions. This sequence is called the multiplication cycle. Kinetic analysis of the various steps requires large numbers of cells in which infection proceeds synchronously i.e. the cells must be infected simultaneously, and secondary infection by progeny virus must be avoided (one-step conditions). (BSc 2nd Year Microbiology Viruses Notes Study Material)
These conditions could be achieved simply in classic studies of Delbruck. The virus is allowed to infect cells for a brief period. Further infection is prevented either by diluting the virus-cell mixture or by adding phage-specific antiserum. The infected cells are then freed from the unabsorbed virus and antiserum by centrifugation. The viral growth cycle can also be studied in individual infected cells isolated in tubes or in small drops of the medium under paraffin oil (single burst experiments). We shall now present details of the one-step multiplication curve of a T2 bacteriophage. (BSc 2nd Year Microbiology Viruses Notes Study Material)
[I] One-step multiplication curve of T2 phage
A one-step multiplication curve describes the production of progeny phage as a function of time after infection under one-step conditions. Bacteria and phage are mixed and adsorption is allowed for 2 minutes: antiphase serum is then added.

The bacteria are recovered by centrifugation and are resuspended in a large volume of the medium at 37° C. A sample is immediately plated to determine the concentration of productive cells. Other samples are taken from time to time and divided into two aliquots; one is shaken with chloroform to disrupt the bacteria and is then assayed for total virus(total titer) e. extracellular plus intracellular virus); the other is freed of bacteria by centrifugation and the supernatant is assayed for the extracellular virus (extracellular titer).
The titers are compared with the concentration of productive cells as 1.0. (BSc 2nd Year Microbiology Viruses Notes Study Material)
These titers are usually expressed as infectious units per productive cell. Plotting their values versus the time of assay yields multiplication curves such as those of the Figure in which the following stages can be recognized:
1. The eclipse period. The total virus titer is almost nil (only residual from the inoculum). The end of the eclipse period is taken as the time at which an average of one infectious unit has been produced for each productive cell.
2. The intracellular accumulation period. Progeny phage accumulates intracellularly but is not yet released into the medium. The end of this period is the time at which one viral infectious unit per productive cell, on average, has appeared extracellularly. The end of this period also marks the end of the latent! period. (BSc Microbiology Viruses Notes Study Material)
3. The rising period. The extracellular phage titer increases until it reaches a constant titer at the end of the multiplication cycle. The average number of infectious units of virus per productive cell at that time represents the viral yield. Progeny phage is released into the medium by the lysis (burst) of each cell, which causes a drop in turbidity of the culture if most cells are infected.
[II] Synthesis of viral macromolecules
Infection of bacteria by T-even phages causes a profound rearrangement of all macromolecular synthesis. The synthesis of cellular DNA stops and is replaced by viral DNA synthesis. The pre-existing cellular DNA breaks down and its components are utilized as precursors of viral DNA. Within a few minutes after infection the synthesis of all cellular DNA, RNA, and protein i.e. that directed by the cellular genome) ceases. Soon the cellular syntheses are entirely replaced by viral syntheses.
This shift represents the fundamental characteristic of viral parasitism: the substitution of viral genes for cellular genes in directing the synthesizing machinery of the cell.
Many enzymes specified by viral genes are synthesized only after infection. These are those causing the shutting off of host RNA synthesis, those causing the cell nucleoid to unfold, and those causing a change in tRNAs, inhibiting the host protein synthesis. The genes introduced into the host cell by the phage DNA are expressed in an orderly temporal sequence. The mechanisms include transcription, translation of viral messengers as well as viral DNA replication. (BSc 2nd Year Microbiology Viruses Notes Study Material)
[III] Maturation and release
Maturation and release are the last two stages of the process of viral multiplication. In maturation the various components become assembled to form complete or mature infectious virions; the release of these leaves the infected cell. In the one-step multiplication curves, the total multiplication curve represents the maturation of the virions; the extracellular curve, and their release.
Replicating viral DNA in a cell forms a single pool from which molecules are withdrawn at random for incorporation into virions. Assembly begins with the aggregation of the soluble capsid proteins. The DNA enters performed empty heads. With T-even phages, several gene products alter the plasma membrane, and then the phage lysozyme (from gene e) crosses the altered membrane and attacks the cell wall, causing lysis. (BSc 2nd Year Microbiology Viruses Notes Study Material)
Genetic recombination. Genetic recombination in T-even phage was discovered in 1946 by Delbruck and by Hershey, who performed genetic crosses between infecting bacteria with a host range (h) and rapid-lysis (r) mutants of phages. The yield of these cultures contained four distinct types of particles; the two parental types and two recombinant types with a marker from each parent. i.e. T2h+r+, T2hr, T2h+ and T2hr+. Genetic maps of phages have been prepared. (BSc 2nd Year Microbiology Viruses Notes Study Material)
Lysogeny and transduction
We have considered above the lytic cycle of a virulent phage. However, the cycle followed by a temperate phage is somewhat different, which involves lysogeny and transduction. We have already referred to these phenomena in relation to the transfer of genetic matter from one bacterial cell to another.
When a sensitive bacterial strain is infected by a temperate phage (a temperate phage, besides multiplying vegetatively and killing the cells at the end of the growth cycle as done by a virulent phage can also produce the phenomenon of lysogeny i.e. the indefinite persistence of phage DNA in the host cells, without phage production), two alternative responses are seen. Some cells are lysed by vegetative multiplication of phage and others are lysogenised. For example, E. coli K 12. (BSc 2nd Year Microbiology Viruses Notes Study Material)
The vegetative cycle of temperate phage (which is involved in transduction) is similar to that of virulent phages but with some modifications. Virions of λ contain a double-stranded linear DNA.47 Kb long, with a complementary single-stranded segment 12 nucleotides long at each 5′ end. The lysogenic state is determined by the activity of the regulatory region of the genome, which both generates immunity and causes the integration of the phage genome in cellular DNA. Repression is demonstrated in Isogenic cells by the absence of vegetative mRNAs. Lysogenic cells contain the immunity repressor but no vegetative proteins.
The basic mechanism in the production and maintenance of the lysogenic state is the antagonism of two repressors – the immunity repressor, and the co-repressor which prevents immunity. The transition of prophage to vegetative phage (induction of lysogenic cell) represents a failure of repression. The induction may be spontaneous or in response to an inducing stimulus. Examples – specialized transducing phages – E.coli K 12 phages gal λ and λ legal and generalized transducing phage- Salmonella phage P 22. The details of transduction are already given.
Protozoal viruses. Virus-like particles have been observed by electron microscopy in Plasmodium spp., including the malarial parasite, in the amoeboflagellate, Naegleria gruberi, the trypanosome Leishmania hertigi and Paramecium aurelia. Diamond and Mattern (1976) demonstrated several viruses infecting the amoeba, Entamoeba histolytica. All viruses of this amoeba are icosahedral. They have also described some filamentous and beaded types of viruses from this amoeba. The headed type had a string of beads about 17 nm in diameter.
Algal viruses. The first presence of a virus in an alga was shown by Safferman and Morris (1963) who isolated it from the blue-green alga Plectonema boryanum. Among the genera of algae infected were Lyngbya, Plectonema, and Phormidium.
The first algal virus was named strain LPP-1, the initials representing these three hosts. From other algae also viruses have been isolated that are similar to LPP-1. These are cyanophages as they have been named from India, Israel, and Scotland. Since 1963, the works on these viruses continued, and at present, about 100 papers have appeared on algal viruses. On marine algae also, similar viruses have been reported.
Morphologically, algal viruses are similar to other viruses. Most of these are icosahedral, somewhat to the Tipula iridescent virus. Some are linear in shape as in TMV. The LPP-1 virus particle is an icosahedron with a hexagonal head capsid; a short tail and double-stranded DNA. A section through algal cells 16 hrs, after infection, shows large numbers of fully formed near-spherical virus particles.
Detailed information on cyanophages is given in a comprehensive review by Padan and Shilo (1973).
Mycophages (Fungal viruses). They have now been reported from over 100 species of fungi including genera from all the main taxonomic groups (Hollings, 1978). Bozarth (1972) estimated that 10-15 percent of randomly sampled fungal isolates examined contained virus-like particles (VLP) in electron microscope examination. Virus infection may be expected in, even more, say some 5000 Fungi.
Fungal viruses have been variously implicated in reducing yields of the cultivated mushroom: increasing the ability of Penicillin chrysogenum to produce antibiotics and reducing the pathogenicity of parasitic fungi. There are some chief features of fungal viruses that contrast markedly with those of plant viruses or bacteriophages. (BSc 2nd Year Microbiology Viruses Notes Study Material)
Most fungal viruses are polyhedral and range from 25 – 45 mm in diameter; almost all contain double-stranded RNA: they seldom causa lysis, though one or two viruses of Penicillium are exceptions; they are transmitted by anastomosis or within spores but not by self-injection nor, it seems by mechanical damage. The viruses of different fungi are serologically unrelated Moreover, no fungal virus has yet been shown to multiply in higher plants although the converse situation is known-e.g. that TMV can multiply in fungi.
Most fungal viruses appear to be latent. They can be detected as membrane-bound, crystalline aggregates within the cytoplasm and sometimes account for up to 0.1 percent of the dry weight of a fungus without causing obvious ill effects. The virus of cultivated mushrooms causes a serious reduction in growth rate and in fruiting capacity.
Some fungi possess cytoplasmically inherited killer factors which may be viruses. There are problems in transmitting viruses from one fungus to another and there are many drawbacks to research in this area. This is the reason why most prefer the use of the term virus-like particle instead of virus for these types of particles. (BSc 2nd Year Microbiology Viruses Notes Study Material)
Mycoplasmal viruses. An excellent review of viruses of mycoplasmas and piroplasms is presented by Maniloff et al (1977). The first virus infecting a mycoplasma was reported by Gourlay (1970) and since then more than 75 viruses! on mycoplasmas have been reported. They have been morphologically and serologically classified into three groups: Group I consists of naked bullet-shaped particles, group 2 roughly spherical enveloped viruses, and Group 3 the polyhedral particles with tails. Most viruses have been isolated from Acholeplasma laidlawii.
All three groups are DNA viruses, in group 1 the DNA is single-stranded covalently closed circular molecule; in group 2 DNA is shown to be double-stranded and that of group 3 is probably also double-stranded in an electron micrograph.
Satellite viruses (Incomplete viruses). Sometimes one virus may depend upon the assistance of another virus in the same cell to help it perform a necessary function of its existence. It has been shown in some viruses of both, plants and animals. One form of such assistance is concerned with vectors, when one virus in a plant may depend upon the presence of another virus to enable it to be transmitted by the aphid vector, a phenomenon called dependent transmission. (BSc 2nd Year Microbiology Viruses Notes Study Material)
The form of dependence on satellite viruses is concerned even more fundamental because it deals with the vital function of replication. In other words, a situation exists in which one virus lacks the power of multiplication without the presence of a second virus to supply the necessary aid. Such a situation is known as a satellite and the incomplete virus is a satellite virus. The virus that allows the satellite virus to infect and multiply is termed an activator and the whole phenomenon is activated. (BSc Microbiology Viruses Notes Study Material)
The first satellite virus to be recorded was found in association with a virus disease of tobacco, tobacco necrosis (Kassanis, 1963).
Both viruses, the complete tobacco necrosis virus (TNV) and the satellite virus (SV) occur together in the roots of the tobacco plant and show no symptoms, and both viruses contain about 20% RNA, TNV, about 28 nm in diameter, often occurs alone in the plant but SV, only 17 nm in diameter has never been found without the presence of TNV. It has been shown that the protein capsid of SV is coded by its own RNA, so the help needed by SV from the activator is not for protein synthesis.
Among animal satellite viruses, one well-known is a Simian adenovirus type 15 (SV15). In preparations besides the typical adenovirus particles, there are also many small hexagonal particles, about 24 nm in diameter. When these small particles were isolated by filtration from the larger particles they failed to produce any ill effect on kidney cells, and they also failed to infect new-borne mice or hamsters. The name given to these small particles was adeno-associated virus (AAV) and they were shown to contain double-stranded DNA.
The typical adenovirus i.e. helper virus was necessary to enable AAV to multiply. Other animal satellite viruses are the Rous sarcoma (RSV) and the Rous-associated virus (RAV) and the Maloney sarcoma virus and its helper. In all these cases, a virus can use for its replication and assembly enzymes and virus protein coded by another virus. One or both viruses may have defective or deficient genes.
A satellite virus, also containing DNA, is phage P4 which is a satellite of phage P2. Unlike most satellite viruses this phage can replicate its nucleic acid in absence of helpers. But it lacks all known genes for phage morphogenesis and its DNA is in the head composed of helper phage proteins. (BSc 2nd Year Microbiology Viruses Notes Study Material)
There are some incomplete viruses in plants and bacteriophages. Several cases are known in which the replication mechanism goes astray with the result that incomplete virus particles are formed. One of the first examples of this type was given by the top and bottom components of the turnip yellow mosaic virus (TYMV). When a purified suspension of this virus is centrifuged at high speeds, two types of particles separate out, both morphologically similar. (BSc 2nd Year Microbiology Viruses Notes Study Material)
The particles at the top of the centrifuge tube contain no nucleic acid and thus are non-infectious whereas the bottom layer contains the complete virus particles which have nucleic acid and are heavier and infectious. There are also a number of different mutants in TMV. Several defective mutants are known for their inability to synthesize protein coats. Three defective mutants are known. One type is defective as it will not reconstitute with TMV nucleic acid to form infectious rods.
There are also satellite RNA viruses, most of which occur in plants. Tobacco necrosis virus (TNV) contains two types of particles in preparation. Of these only the larger is infectious and they are serologically unrelated to smaller satellite particles. SV does not cause any infection. The SV particle contains a single RNA molecule 1200 nucleotides long, which corresponds to a protein 400 amino acids long. SV needs helper TNV for its replication. (BSc 2nd Year Microbiology Viruses Notes Study Material)
Satellite viruses have also been reported in tobacco black ring virus (TBRV) and cucumber mosaic virus.
Slow viruses (Prions). Prions are described as proteinaceous particles thought to cause a number of diseases including slow virus diseases. Prions were named by Stanley B. Prusiner. Prions can survive heat, radiation, and chemical treatments that normally inactivate viruses. They appear to be composed of only proteins. The viruses cause in their hosts a range of infections viz. acute, inapparent, chronic, persistent, latent, slowly progressive, and tumorigenic infections. The so-called slow viruses are involved in slow progressive disease.
The agents which are presumed responsible for slow progressive diseases have been misnamed slow viruses, or also as prions.
Many of these affect the central nervous system and one of the best known is the scrapie agent of sheep and goats which causes the animal to scrape or scratch itself against obstacles. This disease attacks the central nervous system and may take two forms, (1) sometimes extreme sleepiness and (2) more frequently an intense irritation that causes the sheep to rub itself against a gate post or similar object, generally removing some of its wool in the process. It is from this habit the name scrapie was derived. There is a long incubation period of 1-4 years.
The disease is latent and transmitted to sheep in Scotland by means of a vaccine prepared against the disease of louping ill from the brains of sheep carrying latent disease (Andrews, 1967). It is thought to be transmitted to sheep by rams with the latent disease.
The causative agent has not been isolated hence it cannot be positively defined as a virus. Previously, some suspected it to be a viroid. There have been made experimental studies on the relationship between the multiplication of the scrapie agent (in the mouse) and the disease. It has been found that the agent codes for no proteins, there are no antigens against which the immune system can respond. Hence this is a unique situation where the immune response plays no part in the manifestation or suppression of the disease.
There have been many wild and wonderful theories about the nature of the scrapie agent, but there is no doubt that it has heritable properties. However, scrapie agent infectivity is remarkably resistant to inactivation by phenol and UV irradiation which has led to the suggestion that the agent is not a nucleic acid. Recently Stanley Prusiner from the USA has isolated a protein from a scrapie-infected brain which he suggests is the infectious agent. (BSc 2nd Year Microbiology Viruses Notes Study Material)
According to him, these proteins may be able to bring about the production of their own requisite mRNA, and at least for scrapie agents the information is able to flow from protein —> RNA -> DNA thus turning the central dogma of molecular biology on its head. If this is true then prions should have a revolutionary impact on molecular biology since they would constitute the only example to date where genetic information passes from protein to nucleic acid and not the other way around.
However, using this as a probe others have found that scrapie glycoprotein is encoded by DNA found in the brain of normal as well as infected host-the mice. Thus it seems that the expression of the protein is merely enhanced in infected animals and is not in fact the infectious agent. This opinion is turning back to scrapie agent being a small piece of nucleic acid.
The slowly progressive diseases have initial similarities with inapparent infections and later with persistent infections but the late development of the disease symptoms gives them a unique category. Prions are also associated with a few cannibalistic tribes of New Guinea and are transmitted by eating uncooked brains. Another such disease is the Creutzfeld-Jacob disease of humans and animals, similar to scrapie. These all are diseases of the central nervous system. (BSc 2nd Year Microbiology Viruses Notes Study Material)
Some virologists believe that prions are exceptionally small viruses with a capsid so dense that it shields the nucleic acid from research probes. Other virologists are waiting for the isolation of the protein. Current methods to retrieve it from solution to break it down. It is likely that prions use genes in normal animals and activate them to produce more prions. (BSc Microbiology Viruses Notes Study Material)
Oncogenic (Tumor) Viruses
The characteristic tissues of animals are formed by the regulated, limited growth of their component cells. Rarely a cell may escape the normal regulatory processes and divide without restraint, forming an abnormal mass of tissue. Such masses are called tumors or neoplasms.
Some tumors, such as most papillomas (warts) are benign i.e. they remain localized and the animal is unharmed. Other tumors are malignant i.e. their growth is invasive so that the organ is damaged and the animal dies. Often unregulated cells are released from the tumor and establish new neoplastic foci in other parts of the body. The development of malignant tumors is called cancer.
Tumors are usually named by appending the suffix oma to the name of the tissue in which the tumor has arisen. Thus lymphoma is a cancer of the lymphoid tissue, a sarcoma, a cancer of fleshy, non-epithelial tissue; an adenocarcinoma, a cancer of glandular tissue, and so on. The exception is leukemia or cancer of white blood cells. Cancer-causing substances are called carcinogens.
The great majority of viruses in vertebrates are not carcinogenic i.e. they do not have the ability to initiate cancer. The first evidence of a relationship between viruses and cancer was obtained in 1908 when V. Ellerman and O. Bang demonstrated that certain chicken leukemias could be transmitted to healthy birds by cell-free filtrates of diseased blood. The leukemia-causing agent was named a filter passing agent.
This achievement, like that of Iwanowski, passed almost unnoticed. In 1911, P. Rous could similarly transmit a chicken sarcoma, and this also went unnoticed. (BSc 2nd Year Microbiology Viruses Notes Study Material)
Now we know that both the abovesaid diseases are due to carcinogenic viruses. R.E. Shope in 1932 demonstrated a viral origin of rabbit papillomas and the importance was attached to viruses as tumor agents. Bittner (1948) showed that a virus was the cause of mammary cancer in mice.