BSc Microbiology Prokaryotes Other than Eubacteria Notes Study Material
BSc Microbiology Prokaryotes Other than Eubacteria Notes Study Material: BSc is a three-year program in most universities. Some of the universities also offer BSc Honours. After getting enrolled for BSc, there are certain things you require the most to get better grades/marks in BSc. Out of those, there are BSc Study Material, BSc Sample Model Practice Mock Question Answer Papers along with BSc Previous Year Papers. At gurujistudy.com you can easily get all these study materials and notes for free. Here in this post, we are happy to provide BSc 2nd Year Microbiology Prokaryotes Other than Eubacteria Notes Study Material.

BSc Microbiology Prokaryotes Other than Eubacteria Notes Study Material
Archaebacteria
These bacteria differ from true bacteria (eubacteria) in respect of the organization and chemical composition of their cell wall and cell membrane. Their 16S rRNA molecules also differ from other bacteria and ribosomes are insensitive to chloramphenicol. They are also unique in inhabiting extreme environments. Such physiological features coupled with morphological and structural differences make them distinct from true bacteria. They appear to be primitive bacteria and erected to the status of a kingdom, distantly related to other prokaryotes.
They include the methanogens of anaerobic muds; the extreme red halophiles; the salt-loving bacteria of saturated brine and salted fish; and the thermoacidophiles, found in hot sulphur springs or smoldering coal wastes. It is suggested that they represent a very ancient lineage that diverged from the eubacteria very early in the evolutionary process, and have survived only in these specialized ecological niches. They include the following three groups:
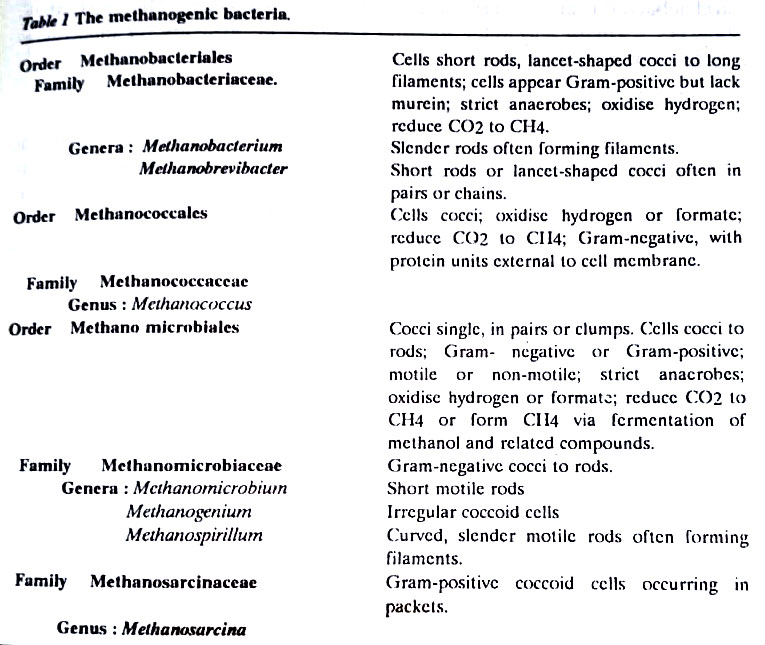
[I] Methane-producing bacteria
They, also known as methanogens represent a highly specialized physiological group. Members of the family Methanobacteriaceae form methane by the reduction of carbon dioxide. They are very strict obligate anaerobes. In order to produce methane, they utilize electrons generated in the oxidation of hydrogen or simple organic compounds, such as acetate and methanol. They are unable to use carbohydrates, proteins, or other complex organic substrates. Methanogens often form consortia in association with other microbes.
Microbes associated with methanogens maintain low oxygen tensions and provide the carbon dioxide and fatty acids required by the methanogens. Such associations are extremely important in the rumen of herbivore animals such as cows. A major source of atmospheric methane is the rumen of these animals. (BSc Microbiology Prokaryotes Other than Eubacteria Notes Study Material)
Methanogenic bacteria may have been among the earliest living organisms on earth because they can grow autotrophically (chemoautotrophs) on hydrogen and carbon dioxide under anaerobic conditions and it is presumed that the early atmosphere of the earth would have permitted the growth of just such organisms. Methanogens can be differentiated on the basis of relatively few morphological and physiological features.
[II] Halobacteriaceae
The genera Halobacterium and Halococcus of this family have the characteristic features of archaebacteria. Halobacterium possesses unusual bacteriorhodopsin-mediated phototrophic metabolism. Besides this unique feature, all members of this family are obligate halophiles, growing only in media containing at least 15% NaCl. They are found in ecosystems having extremely high NaCl concentrations, such as salt lakes, the Dead sea, and foods preserved by salts.
[III] Thermoacidophiles
They include members of the genera like Thermus and Sulpholobus. The members of sulphur-oxidising genus, Sulpholobus on the basis of staining reactions have been characterized as Gram-negative spherical cells. The cell walls of Sulpholobus lack mucin. They also have other features of archaebacteria including rRNA homology and membrane structure. They are thermophiles with an optimum growth temperature of 70 – 750 C. Sulpholobus spp. occur in hot, acidic environments (thermoacidophiles).
Mollicutes (Mycoplasmas)
The common name for this group has traditionally been the Mycoplasmas. However, this usage invites confusion since the word ale refers to the genus, Mycoplasma. It is thus preferred to use the more recently coined term Mollicutes for this group of microorganisms. The distinguishing feature of the mollicutes group of eubacteria is their lack of a defined cell wall. Hence, they are called mollicutes (meaning “soft skin”). (BSc Microbiology Prokaryotes Other than Eubacteria Notes Study Material)
In 1898 the French scientists, E. Nocard and E.R. Roux, studying pleural fluids of cattle suffering from bovine pleuropneumonia, discovered organisms that were entirely different from any other microorganisms known. When cultivated on rich organic media containing about 20% of animal serum, the organisms were found in different forms such as spheroid, thin, branching filaments, stellate or asteroid structures, and other irregular forms. Similar pleomorphic (Gr pleo=many; morphe= forms) organisms were later isolated from other animals such as sheep, goats, dogs, rats mice, and human beings. Similar forms were also found growing as saprophytes in decaying organic matter.
These were named pleuropneumonia-like organisms (PPLO). The species discovered by Nocard and Roux was given the first binomial as Asterococcus mycoides by Borrel et al (1910), meaning rounded and stellate forms with radial, mold-like filaments. It was later on put under the genus Mycoplasma by Nowak (1929) and these organisms are now commonly called mollicutes or mycoplasmas. Characteristic features of the mycoplasmas are as follows:
(1) Unicellular, prokaryotic, usually non-motile, and form fried egg-shaped colonies.
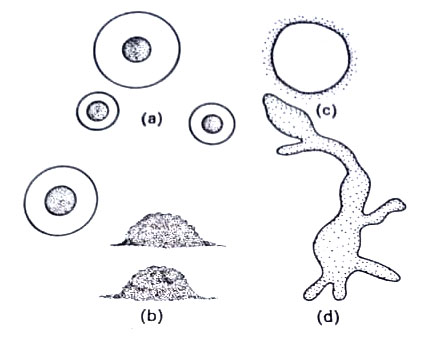
(2) Highly pleomorphic, form varying with culture conditions. Under different microscopy, they appear as small coccoid bodies, ring forms, and fine filaments some of which are branched.
(3) Filtrable through bacterial filters.
(4) Cell wall absent. Cells are delimited by a triple-layered lipoproteinaceous unit membrane, the plasma membrane.
(5) Both DNA and RNA are present. DNA base composition ranges from 23 to 36.
(6) Resistant to antibiotics as penicillins that act on cell walls.
(7) Inhibited by tetracyclines and similar antibiotics that act on metabolic pathways.
(8) Mostly free-living; parasites and saprophytes.
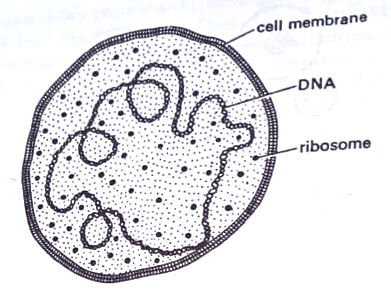
They are true cells. Like animal cells, they have, however, no demonstrable cell walls. The sole remaining structure is the cytoplasmic membrane which like most other cell membranes is phospho-lipid-protein bilayer or unit type, continuous, flexible, and 7-11 nm thick.
Mollicutes are simpler than the cells of higher plants and animals. They possess all the necessary biochemical machinery to grow and multiply in the absence of other cells. The genetic machinery is in the form of DNA, RNA, and ribosomes.
They vary in size mostly from 300 nm to about 0.2 µm in diameter. The volume of the smallest PPLO is about 1×10-3 µm3. The internal structure is typical of prokaryons generally and closely resembles that of true bacteria. The cell membrane encloses the cytoplasm which contains numerous ribosomes, a network of fibrillar DNA, electron-dense areas, ribosomes, soluble RNA, and soluble protein. Nuclear structures, however, are less evident than typical bacteria. Ribosomes are clearly visible and mesosomes are absent.
However, cells of mollicutes divide unevenly into very minute bodies called elementary bodies or minimal reproductive units. They are commonly formed inside large bodies or mature cells. These elementary bodies range in size from about 330 nm to 450 nm. These can pass through bacteria-retaining filters like viruses but are viable on ordinary media. These have a bacteria-like structure. These bodies are often cited as the smallest independently living entities. These represent a stage in the life cycle. They enlarge to form long filaments and mycelia and chains of minute spheres like conidia but are much smaller in size.
It is thought by some that these conidia-like bodies are liberated and that each increases in size to become a larger body several µm in diam, inside which new elementary bodies are formed. These are released by the rupture of the membrane of a larger body. The growth rate of mycoplasma is very rapid, with a generation time of 1-3 hrs. Like viruses and animal cells, mycoplasmas are resistant to penicillin. Many other types of structures are also present. (BSc Microbiology Prokaryotes Other than Eubacteria Notes Study Material)
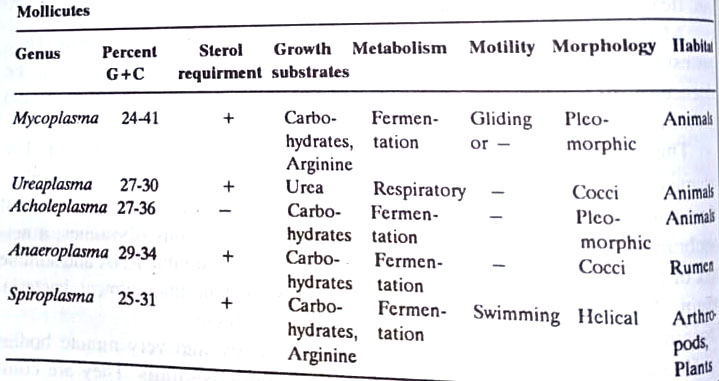
The 16S rRNA sequencing has confirmed that the mollicutes are a coherent phylogenetic group closely related to clostridia. Five genera are recognized in the mollicutes, distinguishing features of which are as follows:
Plant-pathogenic Mollicutes
For many years the yellows of plants were thought to be caused by viruses. In 1967, however, the Japanese workers, Doi et al discovered pleomorphic mycoplasma-like organisms in the phloem cells of plants affected by different yellows-type diseases. Since then MLOs have been found to be associated with over 70 plant diseases that infect over 300 genera of plants. They occur from temperate to tropical regions but it is in the warmer areas that serious losses occur in crops such as coconuts, citrus, rice, maize, cotton, and potatoes.
They also occur in insects and as saprophytes in soil and sewage. A number of mycoplasmal diseases of plants are listed by Ghosh and Raychoudhuri (1972). During the 1940s there occurred an unusual increase in cases of human pneumonia. Monroe Eaton isolated is like an agent from the respiratory tract of patients. This was named Mycoplasma pneumonia (PAP). Mycoplasma hominis causes a sexually-transmitted disease, mycoplasmal urethritis. (BSc Microbiology Prokaryotes Other than Eubacteria Notes Study Material)
L-forms
Klieneberger in 1935 first studied a form of Streptobacillus moniliformis, which was thought to be a symbiotic species of PPLO.
He, while working at the Lister Institute in London, called this form L1, L being the initial of the Lister Institute named for Lord Lister, a pioneer in aseptic surgery. The L1 organism was later shown by Klieneberger and others to be a stable protoplast or L-form of Streptobacillus moniliformis. The two forms interchange under proper growth conditions. L-forms of bacteria are entirely different from L cells, a clone of animal tissue culture cells. Before presenting more information about the L-form of bacteria, we would like to consider in brief the protoplast and spheroplast. (BSc Microbiology Prokaryotes Other than Eubacteria Notes Study Material)
Protoplasts
Some microorganisms, both eukaryotic and prokaryotic (e.g. yeasts, molds, bacteria, some higher plants) that have a distinct cell wall can at times exist without a cell wall in what is designated as a wall-less state. The cell membrane and its intact contents are then called a protoplast. Unless the solute concentration of the suspending fluid is osmotically protective i.e. high enough (e.g. 3-20% glucose; 2-5% NaCl; 10-20% serum) to balance the intracellular osmotic pressure, protoplasts are no longer retained by their thick walls, usually burst. Protoplasts are, therefore, said to be osmotically fragile.
An important property of bacterial cell walls is sensitivity to antibiotic penicillin. Penicillin inhibits the synthesis of peptidoglycans. Young actively growing Gram-positive bacteria are, therefore, sensitive to penicillin. Thus these bacteria can be made protoplasts by treating them with penicillin. Animal cells, including human cells, are unsusceptible to penicillin because there are no peptidoglycans there.
Spheroplasts
Gram-negative bacteria, where the bulk of the cell wall is made by lipopolysaccharides and lipoproteins are usually partly or wholly resistant to penicillin (as it inhibits only peptidoglycan) and also protected by their lipopolysaccharides and lipoproteins of the cell wall (in outer layers) from the action of a group of enzymes, lysozyme, an enzyme discovered by Fleming.
Lysozyme is found in egg white, secretions of skin and mucous membranes, tears, and elsewhere. It attacks superficially the glycosidic bonds in the polysaccharide backbone of peptidoglycan of bacterial cell walls. When Gram-positive bacteria are treated with lysozyme, they are rapidly denuded of their cell walls and become naked protoplasts. In contrast, to make Gram-negative bacteria (though peptidoglycan is present in their cell wall, it is protected by outer layers of lipocomplexes) vulnerable to lysozyme it is necessary first to remove the lipocomplex of the cell wall. These are removed with lipid solvents such as NaOH or ethylenediaminetetraacetate (EDTA).
Even then the wall is not completely removed and the result is osmotically fragile cells, still retaining some remnants of the cell wall. Such only partially denuded cells are called spheroplasts. (BSc Microbiology Prokaryotes Other than Eubacteria Notes Study Material)
Thus protoplasts or spheroplasts may be prepared from various bacteria either by removing the cell wall or by metabolically blocking the synthesis of the cell wall. Lysozyme which dissolves the cell wall and penicillin which inhibits cell wall synthesis is most frequently used in the preparation of protoplasts. Such protoplasts in the hypertonic or isotonic fluid are often able to grow and multiply on agar media.
The colonies resemble the mycoplasmas. These cells without cell walls have been designated as stable L-forms. L-forms can be selected by the cultivation of bacteria on an osmotically buffered penicillin-containing medium. L-forms are, therefore, bacteria in which the primer for peptidoglycan has either been eliminated or modified by penicillin treatment. L-form can be obtained from both Gram-positive and Gram-negative bacteria. Stable L-forms obtained from animals and men are different from mycoplasmas.
Recent genetic studies have shown that L-forms are different from mycoplasma. For example, the DNA base composition of Mycoplasma species ranges from 23 to 36 mole per cent GC, whereas most other bacteria and the L-forms usually have higher percent GC content (63-64 mole per cent GC). (BSc Microbiology Prokaryotes Other than Eubacteria Notes Study Material)
L-forms are formed in Mycobacterium tuberculosis, Streptococcus and others. Some bacteria undergo spontaneous mutation to the loss of cell walls. For example, if DAP is withheld from the medium, which is osmotically protective, the bacteria grow without a cell wall i.e. they are L-forms of the bacteria. (BSc Microbiology Prokaryotes Other than Eubacteria Notes Study Material)
Mycobacteria
Mycobacteria are a group of non-motile rods that are defined on the basis of a distinctive staining property: they are relatively impermeable to various basic dyes, but once stained they retain dyes with tenacity. Specifically, they resist decolorization with acidified organic solvents, and are, therefore, called acid-fast. This property, and their relatively slow growth, is attributed to their lipid-rich cell wall. Mycobacteria range from widespread innocuous saprophytic inhabitants of soil and water to organisms that are responsible for tuberculosis and leprosy.
Tuberculosis is usually confined to internal organs whereas leprosy largely involves the skin. The leprosy bacillus, discovered by Hansen in 1879, was the first bacterium shown to be associated with human disease.
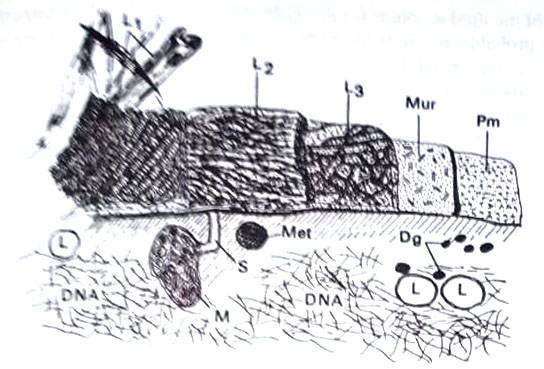
It is strikingly adapted to man. It has been difficult to transfer to any other host, and it has not grown on artificial culture media. In contrast, the agents of human tuberculosis, Mycobacterium tuberculosis, and the closely related M.bovis are readily cultivated on simple media, and are pathogenic to various lower animals, especially guinea pigs and mice. Mycobacteria are considered transitional forms between true bacteria (eubacteria) and Actinomycetales. (BSc Microbiology Prokaryotes Other than Eubacteria Notes Study Material)
- Acid-fastness. To stain tubercle bacillus, in smears or in tissues, the commonly used method is the Ziehl-Neelsen method. The smeared specimen is heated to steaming for 2-3 min. in carbolfuchsin (a mixture of triphenyl-methane dyes rosaniline and pararosaniline in aqueous 5% phenol). Subsequent washing in 95% ethanol – 3% HCl (acid-alcohol) decolorizes most bacteria in a few seconds, whereas acid-fast organisms retain the stain much longer. This property is attributed to the high lipid content in their cell wall. Mycobacteria appear to be Grampositive, but they take up the stain weakly and irregularly and without requiring iodine treatment to retain it.
- Structure. Tubercle bacilli in the animal are typically slightly bent or curved slender rods, about 2 µm – 4 µm long and 0.2 µm – 0.5 µm wide. The rods may be of uniform width but more often appear beaded, with irregularly spaced, unstained vacuoles, or heavily stained knobs. In culture media, the cells may vary from coccoid to filamentous. Strains differ in their tendency to grow as discrete rods or as aggregated long strands, called serpentine cords.
The walls contain peptidoglycan with diaminopimelate, and the cells can be converted to spheroplasts by lysozyme. The walls have a remarkably high lipid content (up to 60% of its dry weight), much of which is attached to polysaccharides. The polysaccharides which include glucan, mannan, arabinogalactan, and arabicomannan, are also found in culture filtrate. The glycolipids and protein are located in a firmly attached outer layer of the wall and the external location of the lipid accounts for the hydrophobic character of the cells.
The lipid-rich wall probably accounts for some of the unusual properties of mycobacteria, like relative impermeability to stains, acid-fastness, unusual resistance to killing by acid or alkali, and resistance to the bactericidal action of antibodies plus complement. Among the lipids, there are true waxes, and glycolipids (mycoses: lipid-soluble compounds with covalently linked lipid and carbohydrate moieties). One type of fatty acid, mycolic acid is unique to the cell walls. These are found in both waxes and glycolipids and are large, saturated, -alkyl, β -hydroxyl fatty acids. (BSc Microbiology Prokaryotes Other than Eubacteria Notes Study Material)
The virulent strains of tubercle bacilli differ from avirulent strains in the nature of their growth. On the surface of a liquid or solid media, virulent strains grow as intertwining serpentine cords, in which the bacilli aggregate with their long axes parallel. Most avirulent strains grow in a more disordered manner. The factor, which may be essential for both virulence and serpentine growth is known as cord factor, which was extracted by Bloch from virulent cells by petroleum ether. The cord factor has been identified as a mycoside, 6,6-dimycolyl trehalose.
It is toxic, inhibiting migration of normal polymorphonuclear leukocytes in vitro (10 µg given to a mouse subcutaneously will kill it). It is more abundant in virulent strains. The toxicity of this factor has been related to many disturbances in microsomal enzymes and mitochondria. and lipid metabolism in mice liver. (BSc Microbiology Prokaryotes Other than Eubacteria Notes Study Material)
- Tuberculin test. Tuberculosis is diagnosed in the laboratory by a tuberculin test. The old method, originally described by Koch, for the preparation of tuberculin is old tuberculin (OT). Here tuberculin is prepared by autoclaving or boiling a culture of tubercle bacilli, concentrating it tenfold on a steam bath, filtering off the debris, and adding glycerol as a preservative. The stock solution retains full potency for years when stored at 5°C. A refined method of tuberculin preparation is purified protein derivative (PPD). It is prepared by precipitation several times with 50% saturated ammonium sulphate. The product is mostly a mixture of small proteins.
Now tuberculin hypersensitivity is tested by intra-dermal injection of 0.1 ml of an appropriate dilution of standardized PPD into the most superficial layers of the skin of the forearm. This is known as the Mantoux test.
The average diameter of induration (and not simply erythema) at the injected site is measured at 48 hr, and reactions are less than 10 mm in diam. are recorded as doubtful. Intense reactions can cause necrosis and scarring. There are other mycobacteria that are nontuberculous and are pathogenic in humans causing other diseases. These are Mycobacterium scrofulaceum, M. kansassii, M. ulcerans, M.marinum, and M. xenopi. (BSc Microbiology Prokaryotes Other than Eubacteria Notes Study Material)
Mycobacterium leprae, (Hansen’s bacillus), the cause of leprosy, grows profusely in lesions, and could not be cultivated in test tubes. In 1960 Shepard discovered that the bacilli could be slowly propagated in the foot pads of mice in a cool environment. Recently nine-banded armadillo is used for skin-test. Antigen, Lepromin, as developed by Mitsuda in 1919, is obtained by boiling human lepromatous tissue rich in bacilli. This material-lepromin is then standardized to contain 160×106 acid-fast bacilli/ml.
Myxobacteria
They are Gram-negative coccus-like or often fusiform rods, 3-12 µm long, producing distinct masses of slime in which they live communally. On solid substrates, they form communal structures that consist of flat, spreading, slimy colonies with lobulated extensions, and are usually called a swarm stage or pseudoplasmodium. (BSc Microbiology Prokaryotes Other than Eubacteria Notes Study Material)
They multiply by binary transverse fission. Under favorable conditions, in the majority of them, the vegetative cells aggregate at a number of points in the inner area of the colony, and fruiting bodies then differentiate from these cellular aggregates.
Each fruit body is made up of slime and bacterial cells; when mature, it acquires a definite size, form, and colour. As the fruiting bodies mature, the cells within them become converted to resting cells, known as myxospores which are frequently referred to as cysts or microcysts. Upon subsequent germination, each myxospore gives rise to a vegetative cell.
These organisms belong to the following three groups:
- Fruiting myxobacteria. They form fruit bodies. The majority of them belong to this group. They are commonly referred to as myxobacteria. DNA base composition ranges from 67 to 71 mole per cent G+C. They are bacteriolytic and cellulolytic. Ex. Myxococcus, Cystobacter, Melittangium, Stigmatella, Polyangium, Chondromyces. In all, except Myxococcus the myxospores are rods. They are spheres in Myxococcus. (BSc Microbiology Prokaryotes Other than Eubacteria Notes Study Material)
- Algicidal nonfruiting myxobacteria. (myxobacteria). The death of natural populations of green algae and cyanobacteria is often caused by myxobacteria. Many of these algicidal forms have not been observed to form fruiting bodies, so their taxonomic position is uncertain. Their DNA base composition is similar to that of fruiting myxobacteria (69 to 71 mole per cent G+C). Consequently, they are referred to by the name of myxobacteria. In contrast to bacteriolytic fruiting bacteria, myxobacteria have simple nutrient requirements. They grow well in liquid media of defined composition and can use glucose or other carbohydrates as sole sources of carbon and nitrogen.
Some kill cyanobacteria by producing extracellular lytic enzymes (protease) that hydrolyze peptide bonds of peptidoglycans of host cell walls.
- Nonfruiting active cellulolytic forms. (the cytophaga group). These gliding bacteria do not form fruiting bodies and they differ markedly from the myxobacteria (including the non-fruiting myxobacteria) in their DNA base composition which lies in the range of 30 to 50 mole per cent G+C. Resting cells are formed only in the genus Sporocytophaga, which produces microcysts, similar to Myxococcus. The other two principal genera are Cytophaga and Flexibacter. The cells are rods, that may also be in chains in Flexibacter.
Most of these are strict aerobes, a few Cytophaga and Flexibacter spp. are facultative anaerobes. The most active aerobic cellulose-decomposing bacteria in soil are certain species of Cytophaga and Sporocytophaga. They can be easily enriched from the soil in a medium with a mineral base containing filter paper as the sole source of carbon and energy. Some species of Cytophaga grow at the expense of chitin and agar. The agarolytic cyanophages are of marine origin, whereas chitinolytic members of the group occur both in soil and in marine waters. (BSc Microbiology Prokaryotes Other than Eubacteria Notes Study Material)
Cellulolytic enzymes are exoenzymes and nondiffusible, thus contact with cellulose substrate is necessary. Therefore cells of cellulolytic forms in culture adhere to cellulose fibers (oriented parallel to the fibers). However, hydrolysis of chitin and agar is mediated by inducible extracellular enzymes, hence contact with the substrate is not necessary. The chitinolytic and agarolytic cyanophages are much less specialized nutritionally than the cellulolytic species. They can also use a wide range of soluble sugars.
Fruiting myxobacteria fall into two nutritional subgroups: bacteriolytic and cellulolytic organisms. The majority of them are bacteriolytic, growing on live or dead cells. Live cells are killed by antibiotics secreted by them. Most myxobacteria do not readily form fruiting bodies on media that support good vegetative growth. Media, poor in nutrients favour fructification.
Life cycle
Myxobacteria produce cysts (resting cells) and fruiting bodies in their life cycle and are hence called fruiting myxobacteria. The vegetative cells are mostly Gram-negative rods, which in some stage of the life cycle undergo alternation of the form: the swarm stage and the encystment or fruiting stage.
- Swarm stage. In this stage, bacteria multiply by fission and secrete a slimy matrix in which they live together. The slime is secreted by the colony as it extends itself, over which the individuals may move or in which they become embedded. The whole structure is coherent and can be stripped intact from the agar surface. This stage lasts from 1-7 days. Encystment then starts.
- Encystment or fruiting stage. Under appropriate conditions, a swarm of vegetative cells gathers together at different points in the slimy matrix and they heap themselves up to form fruiting bodies. Perhaps exhaustion of tyrosine and phenylalanine from medium favors fruiting. Heap may be raised above the substratum. Within a fruiting body or cyst, the rod cells undergo differentiation into shortened, rounded, or dormant resting cells known as myxospores or cysts, or microcysts. The shortened, thick-walled cells in the cyst are usually enveloped by large capsules or gelatinous slime. Myxospores within dried fruiting bodies may remain viable for several years.
Ecologically, many of the myxobacteria are important, occurring as predators of other prokaryotic and eukaryotic organisms. For example, they lyse the cells of true bacteria, blue-green algae, and some higher fungi. The lytic action must be of great importance in the interrelationship of all organisms in the soil, where myxobacteria live in contact with other microorganisms.
Except for a few parasitic species, myxobacteria and cyanophage are of great importance to mankind as scavengers. They are very active in the decomposition of insoluble organic matter such as cellulose, agar, exoskeletal material (chitin) of
insects and crustacea as well as a wide variety of other animal and vegetable matter, thus not only removing waste matters but obligingly transforming them into predigested soluble foods for plants.
Cytophagales
This group of bacteria is grouped with myxobacteria on the basis of their gliding motility on solid surfaces. Myxobacteria and this group are called gliding bacteria. They, unlike myxobacteria, do not produce fruit bodies. Genera in this group show different morphological forms and modes of metabolism. They are unified only by the presence of gliding motion and lack of fruit body formation. Some form filaments and others do not. Some species of Flexibacter may form filaments of up to 100 µm length. Some members are chemolithotrophs.
For instance, Beggiotoa forms filaments that oxidize hydrogen sulphide and deposit sulfur intracellularly when growing on hydrogen sulphide. Cytophaga spp. do not form filaments and their cells contain deep yellow-orange or red pigments and hydrolyze agar, cellulose, and chitin. The presence of hydrolytic enzymes in these bacteria makes them ecologically much important where they are involved in the decomposition of organic matter. (BSc Microbiology Prokaryotes Other than Eubacteria Notes Study Material)
Rickettsiae and Chlamydiae
In 1909, Howard Taylor Ricketts, a pathologist at Univ. of Chicago described a distinctive organism in the blood of victims of Rocky Mountain spotted fever. He also found that the disease could be transmitted along animals by bloodsucking ticks. A year later, Ricketts located a similar organism in the blood of animals infected with Mexican typhus and discovered that in this case, lice were important to transmission. During the course of his work, however, he became infected and fell victim to the disease.
The name rickettsiae were coined to honor the discoverer. Originally, these again were set apart from bacteria, but in the most recent edition of Bergey’s Manual (1974), they have been included as Part 18 in Division II, the Bacteria.
The fundamental differences from ordinary bacteria are (i) a smaller size with a smaller genome providing fewer enzymes (ii) a longer generation time and (ii) a requirement for an exogenous energy supply for growth. They infect ordinarily non-phagocytic cells and are protected from lysosomal degradation by envelopment in a vesicle of the host cell membrane.
Structure
The rickettsiae (sing. rickettsia) measure about 0.3 -0.5 µm in diameter and 0.3 -0.4 µm in length and usually appear as rods with rounded edges, a form known as the Coccobacillus. They also exist in alternate shapes and hence pleomorphic. They are hardly visible under a light microscope. They have no flagella, pili, capsules, or spores. The cell wall is chemically similar to that of Gram-negative bacteria and the cytoplasm contains both DNA and RNA as well as many enzymes. Reproduction is by binary fission.
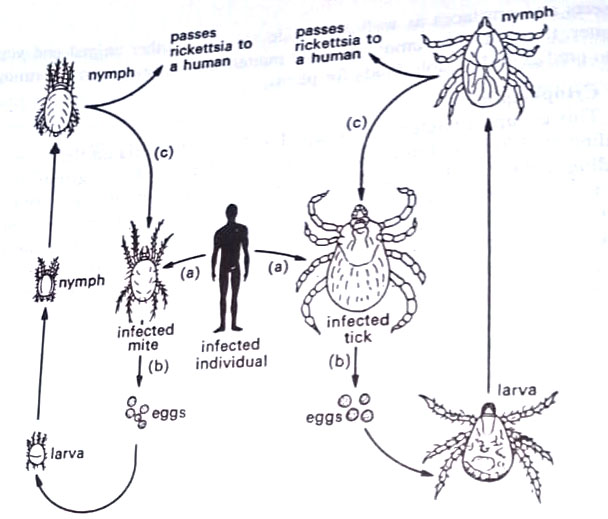
Except for the organism of trench fever, they do not grow on artificial laboratory media. Living tissues such as vertebrate cell cultures, fertilized chicken eggs, or live animals are used for their growth. They are, therefore, obligate intracellular parasites. They are closely associated with arthropods, particularly with arachnids (ticks, mites) and insects (lice, fleas).
Rickettsiae infect both humans and arthropods, the latter serving as vectors. Arthropod is the primary host and humans, are the secondary host (occasionally reverse may occur). Ticks and mites pass the rickettsiae to their young through the eggs in a process called transovarial infection. When the arthropod feeds on the skin of an infected person, it takes a blood meal and becomes infected, particularly along its intestinal tract. As the infected cells of the arthropod burst, the organisms fill the intestinal cavity and are deposited during defecation on the skin of the next individual. (BSc Microbiology Prokaryotes Other than Eubacteria Notes Study Material)
By scratching the site, the person himself inoculates the rickettsiae into the bloodstream, and symptoms of the disease soon appear. The symptoms are a high fever lasting for several days and a skin rash. The rash begins as pink spots called macules. They progress to pink-red spots, like pimples called papules. They soon become dark red and then fade without leaving evidence of scarring. This type of rash is a maculopapular rash. Rickettsial infections usually respond to treatment with tetracyclines or chloramphenicol.
Weil-Felix test
This test is used to identify the presence of rickettsiae without specifying a particular species. A sample of the patient’s blood is first obtained, and the clean serum is separated from the red blood cells. A sample of serum is mixed with the cells of the bacterium, Proteus 0x19. The bacteria will clump together if the serum contains antibodies against the rickettsiae, indicating that a person has the disease or once had it. If no clumping takes place, rickettsial antibodies are probably not present. The Weil-Felix test works because rickettsiae possess antigens, also located in Proteus cells and therefore, both organisms stimulate the immune system to produce the same antibodies.
Chlamydiae
The chlamydiae (sing. chlamydia) are a group of microorganisms presently classified in Part 18 of BM as a type of rickettsiae. These are a subgroup of rickettsiae and are among the smallest recognized bacteria that cause diseases in humans and animals. They measure about 0.25 µm in diameter. These were originally thought of as viruses.
However, later it was found that they possess both DNA and RNA as well as a number of enzymes. They can be treated with some antibiotics. They grow only in living tissues such as fertilized chicken eggs and tissue cultures. They have a complex reproductive cycle that occurs in the cytoplasm of an infected cell. (BSc Microbiology Prokaryotes Other than Eubacteria Notes Study Material)
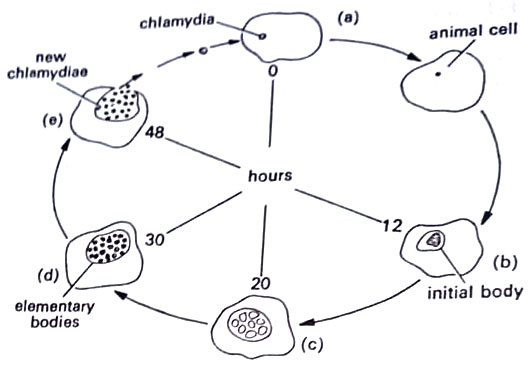
The chlamydia, called an elementary body enlarges to form an initial body or reticulate body that divides by binary fission to form a series of small, dense particles. These develop into highly infectious elementary bodies.
The developmental cycle alternates between two forms: (i) the elementary body (EB), 300 nm in diameter, is specialized for survival when released from the cell, and (ii) the reticulate body (RB) or initial body, up to 1000 nm in diameter and engaged in intracellular multiplication. Within a few hours after entry into the host cells, the EBs begin to undergo profound changes in their cell envelopes and the characteristic central condensate begins to disperse to form a more homogeneous cytoplasm in which strands of nucleic acid and ribosomes are seen.
The resulting RBs continue to grow in size and 10-15 hr. after infection binary fission begins. At 20-30 hr. after infection, some of the RBs develop a central condensation of cytoplasmic contents, decrease in size and become typical EBs.
Most of the RBs, however, continue to multiply until the host cell cytoplasm is almost filled by the colony. Little is known about the mechanism of the release of organisms. (BSc Microbiology Prokaryotes Other than Eubacteria Notes Study Material)
In cell culture, the host cells die and autolyze 40-60 hr. after infection. The process is completed within about 24 hours and the particles may be seen in cells’ cytoplasm as granule-like inclusions, which are characteristic of chlamydial diseases. They are obligate intracellular parasites, like other rickettsiae. (BSc Microbiology Prokaryotes Other than Eubacteria Notes Study Material)
There are only two recognized species of chlamydiae: Chlamydia trachomatis, which causes trachoma in the eyes, and two sexually transmitted diseases, and C. psittaci, the agent of psittacosis, a disease of birds.
Nongonococcal urethritis (Chlamydial urethritis)
Chlamydia trachomatis has more than one strain, collectively called TRIC agent.
TRIC agent is responsible for trachoma (a disease of the eye), inclusion conjunctivitis (also an eye disease), and nongonococcal urethritis (reproductive system disease). When the TRIC agent infects the reproductive system and is passed in sexual contact, the disease is called nongonococcal urethritis (NGU). (BSc Microbiology Prokaryotes Other than Eubacteria Notes Study Material)
This name was coined as the symptoms are remarkably similar to those in gonorrhea (a disease caused by Neisseria gonorrhoeae, a Gram-negative diplococcus, susceptible to penicillin, spectinomycin, and tetracycline). This organism responds only to tetracycline.
Chlamydial urethritis produces symptoms as in gonorrhea, though milder. Females note slight vaginal discharge and inflammation of the cervix. There is burning pain in the urethra. (BSc Microbiology Prokaryotes Other than Eubacteria Notes Study Material)
In a complicated case, fallopian tubes also become involved. In males, there is painful urination and a discharge that is more watery and less copious than in gonorrhea. (BSc Microbiology Prokaryotes Other than Eubacteria Notes Study Material)
Newborn may contract C. trachomatis from an infected mother and develop a disease of eyes known as chlamydial ophthalmia.
BSc Microbiology Prokaryotes Other than Eubacteria Notes Study Material
BSc 2nd Year Sample Model Practice Mock Test Question Answer Papers